当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular dynamics provides new insights into the mechanism of calcium signal transduction and interdomain interactions in cardiac troponin
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-10-21 , DOI: 10.1002/2211-5463.13009 Georgi Z Genchev 1, 2, 3, 4 , Minae Kobayashi 5 , Tomoyoshi Kobayashi 5 , Hui Lu 1, 2, 6
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-10-21 , DOI: 10.1002/2211-5463.13009 Georgi Z Genchev 1, 2, 3, 4 , Minae Kobayashi 5 , Tomoyoshi Kobayashi 5 , Hui Lu 1, 2, 6
Affiliation
|
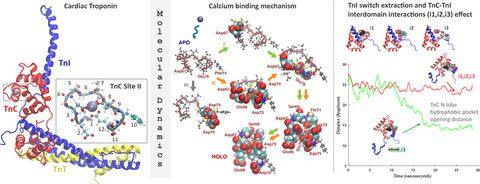
Understanding the regulation of cardiac muscle contraction at a molecular level is crucial for the development of therapeutics for heart conditions. Despite the availability of atomic structures of the protein components of cardiac muscle thin filaments, detailed insights into their dynamics and response to calcium are yet to be fully depicted. In this study, we used molecular dynamics simulations of the core domains of the cardiac muscle protein troponin to characterize the equilibrium dynamics of its calcium-bound and calcium-free forms, with a focus on elements of cardiac muscle contraction activation and deactivation, that is, calcium binding to the cardiac troponin Ca2+-binding subunit (TnC) and the release of the switch region of the troponin inhibitory subunit (TnI) from TnC. The process of calcium binding to the TnC binding site is described as a three-step process commencing with calcium capture by the binding site residues, followed by cooperative residue interplay bringing the calcium ion to the binding site, and finally, calcium–water exchange. Furthermore, we uncovered a set of TnC–TnI interdomain interactions that are critical for TnC N-lobe hydrophobic pocket dynamics. Absence of these interactions allows the closure of the TnC N-lobe hydrophobic pocket while the TnI switch region remains expelled, whereas if the interactions are maintained, the hydrophobic pocket remains open. Modification of these interactions may fine-tune the ability of the TnC N-lobe hydrophobic pocket to close or remain open, modulate cardiac contractility and present potential therapy-relevant targets.
中文翻译:

分子动力学为心肌肌钙蛋白中钙信号转导和域间相互作用的机制提供了新的见解
在分子水平上了解心肌收缩的调节对于开发心脏病治疗方法至关重要。尽管心肌细丝的蛋白质成分的原子结构可用,但对其动力学和对钙的反应的详细了解尚未完全描述。在这项研究中,我们使用心肌蛋白肌钙蛋白核心结构域的分子动力学模拟来表征其钙结合和无钙形式的平衡动力学,重点关注心肌收缩激活和失活的要素,即, 钙与心肌肌钙蛋白 Ca 2+结合结合亚基 (TnC) 和从 TnC 释放肌钙蛋白抑制亚基 (TnI) 的开关区。钙与 TnC 结合位点结合的过程被描述为一个三步过程,首先是结合位点残基捕获钙,然后是协同残基相互作用,将钙离子带到结合位点,最后是钙-水交换。此外,我们发现了一组 TnC-TnI 域间相互作用,这些相互作用对 TnC N-叶疏水袋动力学至关重要。没有这些相互作用允许 TnC N-叶疏水袋关闭,而 TnI 开关区域仍然被排出,而如果保持相互作用,疏水袋保持开放。改变这些相互作用可以微调 TnC N-叶疏水袋关闭或保持打开的能力,
更新日期:2020-10-21
中文翻译:

分子动力学为心肌肌钙蛋白中钙信号转导和域间相互作用的机制提供了新的见解
在分子水平上了解心肌收缩的调节对于开发心脏病治疗方法至关重要。尽管心肌细丝的蛋白质成分的原子结构可用,但对其动力学和对钙的反应的详细了解尚未完全描述。在这项研究中,我们使用心肌蛋白肌钙蛋白核心结构域的分子动力学模拟来表征其钙结合和无钙形式的平衡动力学,重点关注心肌收缩激活和失活的要素,即, 钙与心肌肌钙蛋白 Ca 2+结合结合亚基 (TnC) 和从 TnC 释放肌钙蛋白抑制亚基 (TnI) 的开关区。钙与 TnC 结合位点结合的过程被描述为一个三步过程,首先是结合位点残基捕获钙,然后是协同残基相互作用,将钙离子带到结合位点,最后是钙-水交换。此外,我们发现了一组 TnC-TnI 域间相互作用,这些相互作用对 TnC N-叶疏水袋动力学至关重要。没有这些相互作用允许 TnC N-叶疏水袋关闭,而 TnI 开关区域仍然被排出,而如果保持相互作用,疏水袋保持开放。改变这些相互作用可以微调 TnC N-叶疏水袋关闭或保持打开的能力,









































 京公网安备 11010802027423号
京公网安备 11010802027423号