当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transformation of resident notochord‐descendent nucleus pulposus cells in mouse injury‐induced fibrotic intervertebral discs
Aging Cell ( IF 8.0 ) Pub Date : 2020-10-21 , DOI: 10.1111/acel.13254 Tiffany Y K Au 1, 2 , To-Kam Lam 3 , Yan Peng 3 , Sarah L Wynn 1 , Kenneth M C Cheung 2, 3 , Kathryn S E Cheah 1, 2 , Victor Y L Leung 2, 3
Aging Cell ( IF 8.0 ) Pub Date : 2020-10-21 , DOI: 10.1111/acel.13254 Tiffany Y K Au 1, 2 , To-Kam Lam 3 , Yan Peng 3 , Sarah L Wynn 1 , Kenneth M C Cheung 2, 3 , Kathryn S E Cheah 1, 2 , Victor Y L Leung 2, 3
Affiliation
|
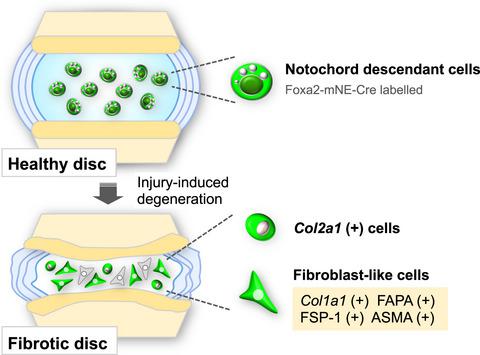
Intervertebral disc degeneration (IDD), a major cause of low back pain, occurs with ageing. The core of the intervertebral disc, the nucleus pulposus (NP), embedded in a proteoglycan‐rich and gelatinous matrix, is derived from the embryonic notochord. With IDD, the NP becomes fibrous, containing fewer cells, which are fibroblastic and of unknown origin. Here, we used a lineage tracing strategy to investigate the origin of cells in the NP in injury‐induced mouse IDD. We established a Foxa2 notochord‐specific enhancer‐driven Cre transgenic mouse model (Foxa2mNE‐Cre) that acts only in the embryonic to foetal period up to E14.5, to genetically label notochord cells with enhanced green fluorescent protein (EGFP). When this mouse is crossed to one carrying a Cre recombinase reporter, Z/EG, EGFP‐labelled NP cells are present even at 2 years of age, consistent with their notochordal origin. We induced tail IDD in Foxa2mNE‐Cre; Z/EG mice by annulus puncture and observed the degenerative changes for 12 weeks. Soon after puncture, EGFP‐labelled NP cells showed strong Col2a1+ expression unlike uninjured control NP. Later, accompanying fibrotic changes, EGFP‐positive NP cells expressed fibroblastic and myofibroblastic markers such as Col1a1, ASMA, FAPA and FSP‐1. The number of EGFP+ cells co‐expressing the fibroblastic markers increased with time after puncture. Our findings suggest resident NP cells initially upregulate Col2a1+ and later transform into fibroblast‐like cells during injury‐mediated disc degeneration and remodelling. This important discovery concerning the cellular origin of fibrotic pathology in injury‐induced IDD has implications for management in disease and ageing.
中文翻译:

小鼠损伤致纤维化椎间盘中驻留脊索下降髓核细胞的转化
椎间盘退变 (IDD) 是导致腰痛的主要原因,随着年龄的增长而发生。椎间盘的核心,髓核(NP),嵌入富含蛋白多糖的凝胶状基质中,来自胚胎脊索。使用 IDD,NP 会变成纤维状,包含较少的成纤维细胞和来源不明的细胞。在这里,我们使用谱系追踪策略来研究损伤诱导的小鼠 IDD 中 NP 中细胞的起源。我们建立了一个Foxa2脊索特异性增强子驱动的 Cre 转基因小鼠模型 (Foxa2mNE-Cre) 仅在胚胎到胎儿期发挥作用,直到 E14.5,用增强的绿色荧光蛋白 (EGFP) 对脊索细胞进行基因标记。当这只小鼠与携带 Cre 重组酶报告基因 Z/EG 的小鼠杂交时,EGFP 标记的 NP 细胞甚至在 2 岁时就存在,与其脊索起源一致。我们在 Foxa2mNE-Cre 中诱导了尾 IDD;Z/EG小鼠通过环穿刺观察退行性变化12周。穿刺后不久,与未受伤的对照 NP 不同,EGFP 标记的 NP 细胞显示出强烈的Col2a1 + 表达。后来,伴随纤维化变化,EGFP 阳性 NP 细胞表达成纤维细胞和肌成纤维细胞标志物,如Col1a1、ASMA、FAPA 和 FSP-1。穿刺后,共表达成纤维细胞标志物的 EGFP+ 细胞数量随时间增加。我们的研究结果表明,常驻 NP 细胞最初上调Col2a1 +,然后在损伤介导的椎间盘退变和重塑过程中转化为成纤维细胞样细胞。这一关于损伤引起的 IDD 中纤维化病理学细胞起源的重要发现对疾病和衰老的管理具有重要意义。
更新日期:2020-11-23
中文翻译:

小鼠损伤致纤维化椎间盘中驻留脊索下降髓核细胞的转化
椎间盘退变 (IDD) 是导致腰痛的主要原因,随着年龄的增长而发生。椎间盘的核心,髓核(NP),嵌入富含蛋白多糖的凝胶状基质中,来自胚胎脊索。使用 IDD,NP 会变成纤维状,包含较少的成纤维细胞和来源不明的细胞。在这里,我们使用谱系追踪策略来研究损伤诱导的小鼠 IDD 中 NP 中细胞的起源。我们建立了一个Foxa2脊索特异性增强子驱动的 Cre 转基因小鼠模型 (Foxa2mNE-Cre) 仅在胚胎到胎儿期发挥作用,直到 E14.5,用增强的绿色荧光蛋白 (EGFP) 对脊索细胞进行基因标记。当这只小鼠与携带 Cre 重组酶报告基因 Z/EG 的小鼠杂交时,EGFP 标记的 NP 细胞甚至在 2 岁时就存在,与其脊索起源一致。我们在 Foxa2mNE-Cre 中诱导了尾 IDD;Z/EG小鼠通过环穿刺观察退行性变化12周。穿刺后不久,与未受伤的对照 NP 不同,EGFP 标记的 NP 细胞显示出强烈的Col2a1 + 表达。后来,伴随纤维化变化,EGFP 阳性 NP 细胞表达成纤维细胞和肌成纤维细胞标志物,如Col1a1、ASMA、FAPA 和 FSP-1。穿刺后,共表达成纤维细胞标志物的 EGFP+ 细胞数量随时间增加。我们的研究结果表明,常驻 NP 细胞最初上调Col2a1 +,然后在损伤介导的椎间盘退变和重塑过程中转化为成纤维细胞样细胞。这一关于损伤引起的 IDD 中纤维化病理学细胞起源的重要发现对疾病和衰老的管理具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号