当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tyrosine in the hinge region of the pore‐forming motif regulates oligomeric β‐barrel pore formation by Vibrio cholerae cytolysin
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-10-21 , DOI: 10.1111/mmi.14631 Anish Kumar Mondal 1 , Paras Verma 1 , Nayanika Sengupta 2 , Somnath Dutta 2 , Shashi Bhushan Pandit 1 , Kausik Chattopadhyay 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-10-21 , DOI: 10.1111/mmi.14631 Anish Kumar Mondal 1 , Paras Verma 1 , Nayanika Sengupta 2 , Somnath Dutta 2 , Shashi Bhushan Pandit 1 , Kausik Chattopadhyay 1
Affiliation
|
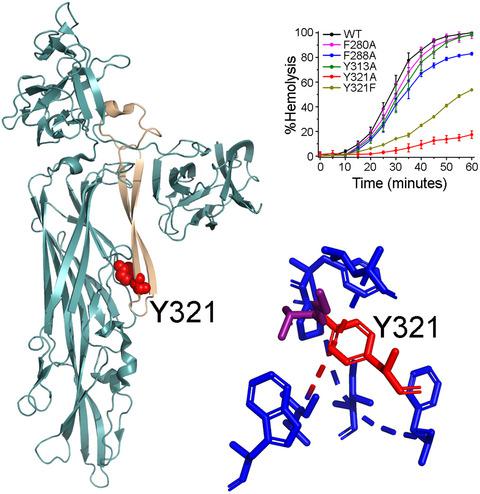
β‐barrel pore‐forming toxins perforate cell membranes by forming oligomeric β‐barrel pores. The most crucial step is the membrane‐insertion of the pore‐forming motifs that create the transmembrane β‐barrel scaffold. Molecular mechanism that regulates structural reorganization of these pore‐forming motifs during β‐barrel pore‐formation still remains elusive. Using Vibrio cholerae cytolysin as an archetypical example of the β‐barrel pore‐forming toxin, we show that a key tyrosine residue (Y321) in the hinge region of the pore‐forming motif plays crucial role in this process. Mutation of Y321 abrogates oligomerization of the membrane‐bound toxin protomers, and blocks subsequent steps of pore‐formation. Our study suggests that the presence of Y321 in the hinge region of the pore‐forming motif is crucial for the toxin molecule to sense membrane‐binding, and to trigger essential structural rearrangements required for the subsequent oligomerization and pore‐formation process. Such a regulatory mechanism of pore‐formation by V. cholerae cytolysin has not been documented earlier in the structurally related β‐barrel pore‐forming toxins.
中文翻译:

成孔基序铰链区中的酪氨酸通过霍乱弧菌溶细胞素调节寡聚 β 桶孔形成
β-桶状成孔毒素通过形成寡聚β-桶状孔来穿透细胞膜。最关键的步骤是膜插入成孔基序,形成跨膜 β 桶支架。在 β 桶状成孔过程中调节这些成孔基序结构重组的分子机制仍然难以捉摸。使用霍乱弧菌溶细胞素作为 β 桶成孔毒素的典型例子,我们表明成孔基序铰链区中的关键酪氨酸残基 (Y321) 在该过程中起着至关重要的作用。Y321 的突变消除了膜结合毒素原体的寡聚化,并阻止了后续的成孔步骤。我们的研究表明,成孔基序铰链区中 Y321 的存在对于毒素分子感知膜结合以及触发后续寡聚化和成孔过程所需的基本结构重排至关重要。这种由霍乱弧菌溶细胞素形成孔的调节机制在结构相关的 β 桶成孔毒素中没有早期记录。
更新日期:2020-10-21
中文翻译:

成孔基序铰链区中的酪氨酸通过霍乱弧菌溶细胞素调节寡聚 β 桶孔形成
β-桶状成孔毒素通过形成寡聚β-桶状孔来穿透细胞膜。最关键的步骤是膜插入成孔基序,形成跨膜 β 桶支架。在 β 桶状成孔过程中调节这些成孔基序结构重组的分子机制仍然难以捉摸。使用霍乱弧菌溶细胞素作为 β 桶成孔毒素的典型例子,我们表明成孔基序铰链区中的关键酪氨酸残基 (Y321) 在该过程中起着至关重要的作用。Y321 的突变消除了膜结合毒素原体的寡聚化,并阻止了后续的成孔步骤。我们的研究表明,成孔基序铰链区中 Y321 的存在对于毒素分子感知膜结合以及触发后续寡聚化和成孔过程所需的基本结构重排至关重要。这种由霍乱弧菌溶细胞素形成孔的调节机制在结构相关的 β 桶成孔毒素中没有早期记录。









































 京公网安备 11010802027423号
京公网安备 11010802027423号