当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First‐principles study of the adsorption mechanism of SO2 and CF4 on the α‐Al2O3 (0001) surface
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-10-22 , DOI: 10.1002/qua.26507 Xinyan Yu 1 , Hongliang Zhang 1 , Jie Li 1 , Hui Guo 1 , Jingkun Wang 1, 2 , Jiacheng Wang 1 , Mengqiu Long 3
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-10-22 , DOI: 10.1002/qua.26507 Xinyan Yu 1 , Hongliang Zhang 1 , Jie Li 1 , Hui Guo 1 , Jingkun Wang 1, 2 , Jiacheng Wang 1 , Mengqiu Long 3
Affiliation
|
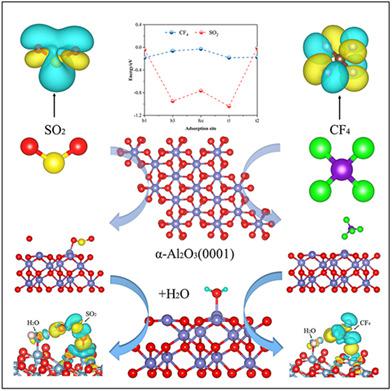
Due to greenhouse gas emission restrictions and environmental protection requirements, the treatment of SO2 and Perfluorinated compound gas has become a problem in the aluminum electrolysis industry. Based on the density functional theory, the adsorption mechanisms of SO2 and CF4 on the surface of clean α‐Al2O3 (0001) were studied. Using projected augmented wave/Perdew‐Burke‐Ernzerhof for calculation, it can be found that SO2 is adsorbed to form an AlO1SO2 structure, and the most stable adsorption energy is −1.52 eV. The adsorption of SO2 is mainly due to the action of π‐p orbitals and the partial contribution of sp2 hybrid orbitals and s orbitals. The most stable adsorption energy of CF4 is −0.38 eV, and it is difficult to transfer charge on the surface. Overall, SO2 is chemisorbed on the clean surface, while CF4 is physisorbed, and their coadsorption with H2O is disadvantageous to the adsorption reaction. These results are very important for understanding the adsorption mechanism of CF4 and SO2 on alumina.
中文翻译:

第一性原理研究SO2-和CF4在α-Al2O3(0001)表面上的吸附机理
由于温室气体排放限制和环境保护要求,SO 2和全氟化合物气体的处理已成为铝电解工业中的问题。基于密度泛函理论上,SO的吸附机制2和CF 4的清洁的表面上的α-Al 2 ö 3(0001)进行了研究。使用投影缀波/ Perdew-伯克Ernzerhof进行计算,可以发现,SO 2被吸附至形成Al Ò 1 小号 Ò 2结构,和最稳定的吸附能是-1.52电子伏特。SO 2的吸附这主要是由于π-p轨道的作用以及sp 2混合轨道和s轨道的部分贡献。CF 4的最稳定的吸附能为-0.38 eV,并且难以在表面上转移电荷。总之,SO 2被化学吸附在干净的表面上,而CF 4被物理吸附,它们与H 2 O的共吸附对吸附反应不利。这些结果对于理解CF 4和SO 2在氧化铝上的吸附机理非常重要。
更新日期:2020-10-22
中文翻译:

第一性原理研究SO2-和CF4在α-Al2O3(0001)表面上的吸附机理
由于温室气体排放限制和环境保护要求,SO 2和全氟化合物气体的处理已成为铝电解工业中的问题。基于密度泛函理论上,SO的吸附机制2和CF 4的清洁的表面上的α-Al 2 ö 3(0001)进行了研究。使用投影缀波/ Perdew-伯克Ernzerhof进行计算,可以发现,SO 2被吸附至形成Al Ò 1 小号 Ò 2结构,和最稳定的吸附能是-1.52电子伏特。SO 2的吸附这主要是由于π-p轨道的作用以及sp 2混合轨道和s轨道的部分贡献。CF 4的最稳定的吸附能为-0.38 eV,并且难以在表面上转移电荷。总之,SO 2被化学吸附在干净的表面上,而CF 4被物理吸附,它们与H 2 O的共吸附对吸附反应不利。这些结果对于理解CF 4和SO 2在氧化铝上的吸附机理非常重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号