当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Why Does the Novel Coronavirus Spike Protein Interact so Strongly with the Human ACE2? A Thermodynamic Answer
ChemBioChem ( IF 2.6 ) Pub Date : 2020-10-20 , DOI: 10.1002/cbic.202000455 Jones Andrade 1 , Paulo Fernando Bruno Gonçalves 1 , Paulo Augusto Netz 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-10-20 , DOI: 10.1002/cbic.202000455 Jones Andrade 1 , Paulo Fernando Bruno Gonçalves 1 , Paulo Augusto Netz 1
Affiliation
|
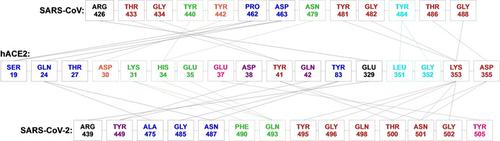
The receptor binding domain (RBD) from coronavirus’ spike protein recognizes human angiotensin‐converting enzyme 2 (hACE2), making it an important drug target. This in silico study compares RBD interactions from SARS‐CoV and SARS‐CoV‐2 with the hACE2, revealing an intricate pattern of hydrogen bonds and hydrophobic interactions, and a free energy of binding consistently stronger for the later virus.

中文翻译:

为什么新型冠状病毒刺突蛋白与人ACE2如此强烈地相互作用?热力学答案
冠状病毒刺突蛋白的受体结合域(RBD)识别人血管紧张素转换酶2(hACE2),使其成为重要的药物靶标。这项计算机模拟研究将SARS-CoV和SARS-CoV-2的RBD相互作用与hACE2进行了比较,揭示了复杂的氢键和疏水相互作用模式,并且结合自由能对于后一种病毒更强。

更新日期:2020-10-20

中文翻译:

为什么新型冠状病毒刺突蛋白与人ACE2如此强烈地相互作用?热力学答案
冠状病毒刺突蛋白的受体结合域(RBD)识别人血管紧张素转换酶2(hACE2),使其成为重要的药物靶标。这项计算机模拟研究将SARS-CoV和SARS-CoV-2的RBD相互作用与hACE2进行了比较,揭示了复杂的氢键和疏水相互作用模式,并且结合自由能对于后一种病毒更强。












































 京公网安备 11010802027423号
京公网安备 11010802027423号