当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MeOTf‐Catalyzed Intramolecular Acyl‐Cyclization of Aryl Isocyanates: Efficient Access to Phenanthridin‐6(5H)‐one and 3,4‐Dihydroisoquinolin‐1(2H)‐one Derivatives
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-10-21 , DOI: 10.1002/ajoc.202000556 Song Zou 1 , Zeyu Zhang 1 , Chao Chen 1 , Chanjuan Xi 1, 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-10-21 , DOI: 10.1002/ajoc.202000556 Song Zou 1 , Zeyu Zhang 1 , Chao Chen 1 , Chanjuan Xi 1, 2
Affiliation
|
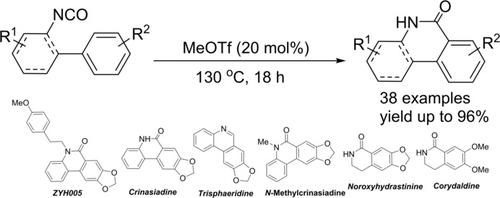
MeOTf‐catalyzed intramolecular acyl‐cyclization of aryl isocyanates has been realized to provide (NH)‐phenanthridinones and lactams under metal‐free conditions. A variety of functional groups are tolerated in their scaffolds with good to excellent yields. The reaction could be carried to gram scale and a range of natural alkaloids, such as crinasiadine, benzophenidine alkaloid (ZYH005), N‐methylcrinasiadine, and trisphaeridine could be synthesized directly or in short steps with high yields.
中文翻译:

MeOTf催化的芳基异氰酸酯的分子内酰基环化反应:有效地获得对菲啶-6(5H)-1和3,4-二氢异喹啉-1(2H)-1衍生物
MeOTf催化的芳基异氰酸酯分子内酰基环化反应可在无金属条件下提供(NH)-菲啶酮和内酰胺。各种功能基团在其支架中均具有良好至极佳的产量。该反应可以进行克量级的合成,可以直接或在短时间内以高收率合成一系列天然生物碱,例如克林纳西丁,苯甲酰苯碱生物碱(ZYH005),N-甲基肉桂酸和三叶啶。
更新日期:2020-10-21
中文翻译:

MeOTf催化的芳基异氰酸酯的分子内酰基环化反应:有效地获得对菲啶-6(5H)-1和3,4-二氢异喹啉-1(2H)-1衍生物
MeOTf催化的芳基异氰酸酯分子内酰基环化反应可在无金属条件下提供(NH)-菲啶酮和内酰胺。各种功能基团在其支架中均具有良好至极佳的产量。该反应可以进行克量级的合成,可以直接或在短时间内以高收率合成一系列天然生物碱,例如克林纳西丁,苯甲酰苯碱生物碱(ZYH005),N-甲基肉桂酸和三叶啶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号