当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Commercialization of an End-to-End Continuous Pharmaceutical Production Process: A Pilot Plant Case Study
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.oprd.0c00383 Christopher J. Testa 1 , Chuntian Hu 1 , Khrystyna Shvedova 1 , Wei Wu 1 , Ridade Sayin 1 , Federica Casati 1, 2 , Bhakti S. Halkude 1 , Paul Hermant 1 , Dongying Erin Shen 1 , Anjana Ramnath 1 , Qinglin Su 1 , Stephen C. Born 1 , Bayan Takizawa 1 , Saptarshi Chattopadhyay 1 , Thomas F. O’Connor 3 , Xiaochuan Yang 3 , Sukumar Ramanujam 4 , Salvatore Mascia 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.oprd.0c00383 Christopher J. Testa 1 , Chuntian Hu 1 , Khrystyna Shvedova 1 , Wei Wu 1 , Ridade Sayin 1 , Federica Casati 1, 2 , Bhakti S. Halkude 1 , Paul Hermant 1 , Dongying Erin Shen 1 , Anjana Ramnath 1 , Qinglin Su 1 , Stephen C. Born 1 , Bayan Takizawa 1 , Saptarshi Chattopadhyay 1 , Thomas F. O’Connor 3 , Xiaochuan Yang 3 , Sukumar Ramanujam 4 , Salvatore Mascia 1
Affiliation
|
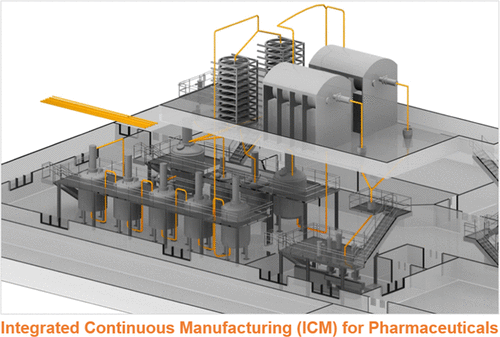
The pharmaceutical industry faces multiple challenges (e.g., inefficient manufacturing techniques, quality control issues, and supply chain vulnerabilities) because of its current batch-wise approach to manufacturing. Recent regulatory support for continuous manufacturing and advances in continuous process technologies have caused an increase in interest from some drug manufacturers to modernize their production processes. However, many of these companies have focused on hybrid processes, where only certain steps are continuous, while others remain batch. Herein, the quality by design (QbD)-based design strategy and operation of an end-to-end integrated continuous manufacturing (ICM) pilot plant that produces both small-molecule active pharmaceutical ingredient (API) and oral solid dosages (OSDs) are discussed. Additionally, important quality and economic matters pertaining to scale-up and commercialization are addressed. ICM has significant benefits, including better quality control, increased supply chain flexibility, a lower capital investment (in the example provided, a ∼ 90% reduction), and lower operating costs (in the example provided, a 33.6% reduction for API and 29.4% reduction for tablets).
中文翻译:

端到端连续药物生产过程的设计和商业化:中试工厂案例研究
制药行业由于其目前的分批生产方式而面临多重挑战(例如,低效的制造技术,质量控制问题和供应链漏洞)。最近对连续生产的监管支持和连续过程技术的进步,引起一些药物制造商对现代化生产过程的兴趣增加。但是,这些公司中的许多公司都专注于混合流程,其中只有某些步骤是连续的,而其他步骤则是分批的。在此,基于质量(QbD)的设计策略和生产小分子活性药物成分(API)和口服固体剂量(OSD)的端到端集成连续制造(ICM)中试工厂的运行情况如下:讨论过。另外,解决了与规模化和商业化有关的重要质量和经济问题。ICM具有显着的优势,包括更好的质量控制,增强的供应链灵活性,更低的资本投资(在提供的示例中,降低了约90%),并降低了运营成本(在提供的示例中,API降低了33.6%,片剂降低了29.4%)。
更新日期:2020-12-18
中文翻译:

端到端连续药物生产过程的设计和商业化:中试工厂案例研究
制药行业由于其目前的分批生产方式而面临多重挑战(例如,低效的制造技术,质量控制问题和供应链漏洞)。最近对连续生产的监管支持和连续过程技术的进步,引起一些药物制造商对现代化生产过程的兴趣增加。但是,这些公司中的许多公司都专注于混合流程,其中只有某些步骤是连续的,而其他步骤则是分批的。在此,基于质量(QbD)的设计策略和生产小分子活性药物成分(API)和口服固体剂量(OSD)的端到端集成连续制造(ICM)中试工厂的运行情况如下:讨论过。另外,解决了与规模化和商业化有关的重要质量和经济问题。ICM具有显着的优势,包括更好的质量控制,增强的供应链灵活性,更低的资本投资(在提供的示例中,降低了约90%),并降低了运营成本(在提供的示例中,API降低了33.6%,片剂降低了29.4%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号