当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Green and Sustainable Manufacturing Process for Gefapixant Citrate (MK-7264) Part 3: Development of a One-Pot Formylation–Cyclization Sequence to the Diaminopyrimidine Core
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-10-20 , DOI: 10.1021/acs.oprd.0c00246 Kallol Basu 1 , Dan Lehnherr 1 , Gary E. Martin 2 , Richard A. Desmond 1 , Yu-hong Lam 3 , Feng Peng 1 , John Y. L. Chung 1 , Rebecca A. Arvary 1 , Michael A. Zompa 1 , Si-Wei Zhang 1 , Jinchu Liu 1 , Zachary E. X. Dance 4 , Patrick Larpent 1 , Ryan D. Cohen 2 , Francisco J. Guzman 1 , Nicholas J. Rogus 1 , Michael J. Di Maso 1 , Hong Ren 1 , Kevin M. Maloney 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-10-20 , DOI: 10.1021/acs.oprd.0c00246 Kallol Basu 1 , Dan Lehnherr 1 , Gary E. Martin 2 , Richard A. Desmond 1 , Yu-hong Lam 3 , Feng Peng 1 , John Y. L. Chung 1 , Rebecca A. Arvary 1 , Michael A. Zompa 1 , Si-Wei Zhang 1 , Jinchu Liu 1 , Zachary E. X. Dance 4 , Patrick Larpent 1 , Ryan D. Cohen 2 , Francisco J. Guzman 1 , Nicholas J. Rogus 1 , Michael J. Di Maso 1 , Hong Ren 1 , Kevin M. Maloney 1
Affiliation
|
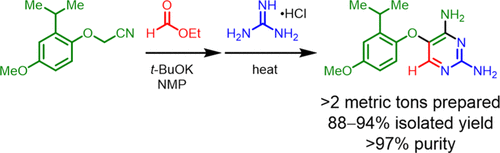
The development of a safe, robust, and efficient manufacturing route for the synthesis of diaminopyrimidine 1, a key intermediate to gefapixant citrate (MK-7264), is described. A full mechanistic understanding of the cyclization step in the presence of guanidine was established by performing isotopic labeling experiments and identification of impurities. Guided by the mechanistic understanding, further attempts to modify the cyclization reaction by employing additives to reduce the triazine (9) formation and guanidine loading will also be presented. This newly developed method delivered compound 1 in 88–94% yield on a commercial scale and addressed the shortcomings of the early synthetic route including high PMI, low atom economy, long cycle-time, and multiple purifications to achieve the desired quality.
中文翻译:

柠檬酸Gefapixant(MK-7264)的绿色可持续制造工艺开发,第3部分:二氨基嘧啶核的一锅式甲酰化-环化序列的开发
描述了开发安全,可靠和高效的生产路线的方法,以合成柠檬酸吉法pixant(MK-7264)的关键中间体二氨基嘧啶1。通过进行同位素标记实验和鉴定杂质,建立了在胍存在下环化步骤的完整机理的认识。在机械理解的指导下,还将提出通过使用添加剂减少三嗪(9)形成和胍负载量来修饰环化反应的进一步尝试。这种新开发的方法可提供化合物1 以商业规模获得88-94%的收率,并解决了早期合成路线的缺点,包括高PMI,低原子经济性,长循环时间和多次纯化以达到所需质量。
更新日期:2020-11-21
中文翻译:

柠檬酸Gefapixant(MK-7264)的绿色可持续制造工艺开发,第3部分:二氨基嘧啶核的一锅式甲酰化-环化序列的开发
描述了开发安全,可靠和高效的生产路线的方法,以合成柠檬酸吉法pixant(MK-7264)的关键中间体二氨基嘧啶1。通过进行同位素标记实验和鉴定杂质,建立了在胍存在下环化步骤的完整机理的认识。在机械理解的指导下,还将提出通过使用添加剂减少三嗪(9)形成和胍负载量来修饰环化反应的进一步尝试。这种新开发的方法可提供化合物1 以商业规模获得88-94%的收率,并解决了早期合成路线的缺点,包括高PMI,低原子经济性,长循环时间和多次纯化以达到所需质量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号