当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Resonance-Enhanced Charge Delocalization in Carbazole–Oligoyne–Oxadiazole Conjugates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/jacs.0c04003 Anna Zieleniewska 1 , Xiaotao Zhao 2 , Stefan Bauroth 1 , Changsheng Wang 2 , Andrei S. Batsanov 2 , Christina Krick Calderon 1 , Axel Kahnt 3 , Timothy Clark 4 , Martin R. Bryce 2 , Dirk M. Guldi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/jacs.0c04003 Anna Zieleniewska 1 , Xiaotao Zhao 2 , Stefan Bauroth 1 , Changsheng Wang 2 , Andrei S. Batsanov 2 , Christina Krick Calderon 1 , Axel Kahnt 3 , Timothy Clark 4 , Martin R. Bryce 2 , Dirk M. Guldi 1
Affiliation
|
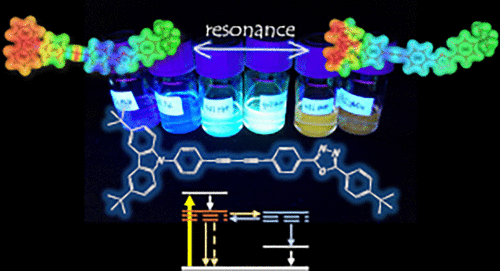
There are notably few literature reports of electron donor-acceptor oligoynes, even though they offer unique opportunities for studying charge transport through "all-carbon" molecular bridges. In this context, the current study focuses on a series of carbazole-(C≡C)n-2,5-diphenyl-1,3,4-oxadiazoles (n = 1-4) as conjugated π-systems in general and explores their photophysical properties in particular. Contrary to the behavior of typical electron donor-acceptor systems, for these oligoynes, the rates of charge recombination after photoexcitation increase with increasing electron donor-acceptor distance. To elucidate this unusual performance, we conducted detailed photophysical and time-dependent density functional theory investigations. Significant delocalization of the molecular orbitals along the bridge indicates that the bridging states come into resonance with either the electron donor or acceptor, thereby accelerating the charge transfer. Moreover, the calculated bond lengths reveal a reduction in bond-length alternation upon photoexcitation, indicating significant cumulenic character of the bridge in the excited state. In short, strong vibronic coupling between the electron-donating N-arylcarbazoles and the electron-accepting 1,3,4-oxadiazoles accelerates the charge recombination as the oligoyne becomes longer.
更新日期:2020-10-21











































 京公网安备 11010802027423号
京公网安备 11010802027423号