当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroless Production of Fertilizer (Struvite) and Hydrogen from Synthetic Agricultural Wastewaters
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/jacs.0c07916 László Kékedy-Nagy 1 , Mojtaba Abolhassani 1 , Sergio I. Perez Bakovic 1 , Zahra Anari 1 , John P. Moore II 1 , Bruno G. Pollet 2 , Lauren F. Greenlee 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/jacs.0c07916 László Kékedy-Nagy 1 , Mojtaba Abolhassani 1 , Sergio I. Perez Bakovic 1 , Zahra Anari 1 , John P. Moore II 1 , Bruno G. Pollet 2 , Lauren F. Greenlee 1
Affiliation
|
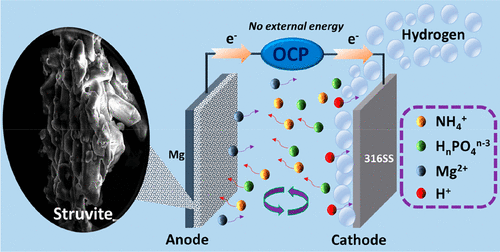
The drive toward sustainable phosphorus (P) recovery from agricultural and municipal wastewater streams has intensified. However, combining P recovery with energy conservation is perhaps one of the greatest challenges of this century. In this study, we report for the first time the simultaneous electroless production of struvite and dihydrogen from aqueous ammonium dihydrogen phosphate (NH4H2PO4) solutions in contact with either a pure magnesium (Mg) or a Mg alloy as the anode and 316 stainless steel (SS) as the cathode placed in a bench-scale electrochemical reactor. During the electroless process (i.e., in the absence of external electrical power), the open circuit potential (OCP), the formation of struvite on the anode, and the generation of dihydrogen at the cathode were monitored. We found that struvite is formed, and that struvite crystal structure/morphology and precipitate film thickness are affected by the concentration of the HnPO4n-3/NH4+ in solution and the composition of the anode. The pure Mg anode produced a porous 0.6-4.1 μm thick film, while the AZ31 Mg alloy produced a more compact 1.7-9.9 μm thick struvite film. Kinetic analyses revealed that Mg dissolution to Mg2+ followed mostly a zero-order kinetic rate law for both Mg anode materials, and the rate constants (k) depended upon the struvite layer morphology. Fourier-transform infrared spectrometry, X-ray diffraction, and scanning electron microscopy indicated that the synthesized struvite was of high quality. The dihydrogen and Mg2+ in solution were detected by a gas chromatography-thermal conductivity detector and ion chromatography, respectively. Furthermore, we fully demonstrate that the reactor was able to remove ∼73% of the HnPO4n-3 present in a natural poultry wastewater as mainly struvite. This study highlights the feasibility of simultaneously producing struvite and dihydrogen from wastewater effluents with no energy input in a green and sustainable approach.
中文翻译:

从合成农业废水中化学法生产肥料(鸟粪石)和氢气
从农业和城市废水流中实现可持续磷 (P) 回收的驱动力已经加强。然而,将磷回收与节能相结合可能是本世纪最大的挑战之一。在这项研究中,我们首次报告了从磷酸二氢铵 (NH4H2PO4) 水溶液与纯镁 (Mg) 或镁合金作为阳极和 316 不锈钢 (SS ) 作为放置在实验室规模电化学反应器中的阴极。在无电过程中(即在没有外部电源的情况下),对开路电位 (OCP)、阳极上鸟粪石的形成和阴极上双氢的产生进行了监测。我们发现鸟粪石形成了,鸟粪石晶体结构/形态和沉淀膜厚度受溶液中 HnPO4n-3/NH4+ 的浓度和阳极组成的影响。纯镁阳极产生 0.6-4.1 微米厚的多孔膜,而 AZ31 镁合金产生更致密的 1.7-9.9 微米厚的鸟粪石膜。动力学分析表明,对于两种镁阳极材料,镁溶解到 Mg2+ 主要遵循零级动力学速率定律,并且速率常数 (k) 取决于鸟粪石层形态。傅里叶变换红外光谱、X 射线衍射和扫描电子显微镜表明合成的鸟粪石是高质量的。溶液中的二氢和Mg2+分别用气相色谱-热导检测器和离子色谱检测。此外,我们充分证明了反应器能够去除天然家禽废水中约 73% 的 HnPO4n-3,主要是鸟粪石。这项研究强调了以绿色和可持续的方法从废水中同时生产鸟粪石和二氢的可行性,无需能源投入。
更新日期:2020-10-21
中文翻译:

从合成农业废水中化学法生产肥料(鸟粪石)和氢气
从农业和城市废水流中实现可持续磷 (P) 回收的驱动力已经加强。然而,将磷回收与节能相结合可能是本世纪最大的挑战之一。在这项研究中,我们首次报告了从磷酸二氢铵 (NH4H2PO4) 水溶液与纯镁 (Mg) 或镁合金作为阳极和 316 不锈钢 (SS ) 作为放置在实验室规模电化学反应器中的阴极。在无电过程中(即在没有外部电源的情况下),对开路电位 (OCP)、阳极上鸟粪石的形成和阴极上双氢的产生进行了监测。我们发现鸟粪石形成了,鸟粪石晶体结构/形态和沉淀膜厚度受溶液中 HnPO4n-3/NH4+ 的浓度和阳极组成的影响。纯镁阳极产生 0.6-4.1 微米厚的多孔膜,而 AZ31 镁合金产生更致密的 1.7-9.9 微米厚的鸟粪石膜。动力学分析表明,对于两种镁阳极材料,镁溶解到 Mg2+ 主要遵循零级动力学速率定律,并且速率常数 (k) 取决于鸟粪石层形态。傅里叶变换红外光谱、X 射线衍射和扫描电子显微镜表明合成的鸟粪石是高质量的。溶液中的二氢和Mg2+分别用气相色谱-热导检测器和离子色谱检测。此外,我们充分证明了反应器能够去除天然家禽废水中约 73% 的 HnPO4n-3,主要是鸟粪石。这项研究强调了以绿色和可持续的方法从废水中同时生产鸟粪石和二氢的可行性,无需能源投入。











































 京公网安备 11010802027423号
京公网安备 11010802027423号