当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transmembrane Ion Channels Formed by a Star of David [2]Catenane and a Molecular Pentafoil Knot
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/jacs.0c07977 David P August 1 , Stefan Borsley 1 , Scott L Cockroft 2 , Flavio Della Sala 1, 3 , David A Leigh 1 , Simon J Webb 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/jacs.0c07977 David P August 1 , Stefan Borsley 1 , Scott L Cockroft 2 , Flavio Della Sala 1, 3 , David A Leigh 1 , Simon J Webb 1, 3
Affiliation
|
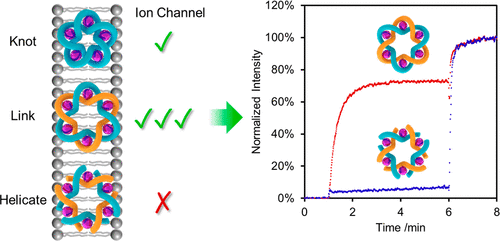
A (FeII)6-coordinated triply interlocked (“Star of David”) [2]catenane (612 link) and a (FeII)5-coordinated pentafoil (51) knot are found to selectively transport anions across phospholipid bilayers. Allostery, topology, and building block stoichiometry all play important roles in the efficacy of the ionophoric activity. Multiple FeII cation coordination by the interlocked molecules is crucial: the demetalated catenane exhibits no anion binding in solution nor any transmembrane ion transport properties. However, the topologically trivial, Lehn-type cyclic hexameric FeII helicates—which have similar anion binding affinities to the metalated Star of David catenane in solution—also display no ion transport properties. The unanticipated difference in behavior between the open- and closed-loop structures may arise from conformational restrictions in the linking groups that likely enhances the rigidity of the channel-forming topologically complex molecules. The (FeII)6-coordinated Star of David catenane, derived from a hexameric cyclic helicate, is 2 orders of magnitude more potent in terms of ion transport than the (FeII)5-coordinated pentafoil knot, derived from a cyclic pentamer of the same building block. The reduced efficacy is reminiscent of multisubunit protein ion channels assembled with incorrect monomer stoichiometries.
中文翻译:

由大卫之星[2]链烷和分子五叶结形成的跨膜离子通道
发现 (FeII)6 配位三重互锁(“大卫之星”)[2]链烯(612 连接)和 (FeII)5 配位五箔 (51) 结选择性地跨磷脂双层传输阴离子。变构、拓扑和结构单元化学计量都在离子载体活性的功效中发挥着重要作用。互锁分子的多个 FeII 阳离子配位至关重要:脱金属的索烯在溶液中不表现出阴离子结合,也不表现出任何跨膜离子传输特性。然而,拓扑平凡的 Lehn 型环状六聚 FeII 螺旋结构(与溶液中的金属化大卫之星索烯具有相似的阴离子结合亲和力)也没有表现出离子传输特性。开环和闭环结构之间的行为差异可能是由连接基团的构象限制引起的,这可能增强了通道形成拓扑复杂分子的刚性。衍生自六聚环状螺旋的 (FeII)6 配位大卫星索烷在离子传输方面比衍生自相同环状五聚体的 (FeII)5 配位五叶结强 2 个数量级积木。功效降低让人想起用不正确的单体化学计量组装的多亚基蛋白质离子通道。
更新日期:2020-10-21
中文翻译:

由大卫之星[2]链烷和分子五叶结形成的跨膜离子通道
发现 (FeII)6 配位三重互锁(“大卫之星”)[2]链烯(612 连接)和 (FeII)5 配位五箔 (51) 结选择性地跨磷脂双层传输阴离子。变构、拓扑和结构单元化学计量都在离子载体活性的功效中发挥着重要作用。互锁分子的多个 FeII 阳离子配位至关重要:脱金属的索烯在溶液中不表现出阴离子结合,也不表现出任何跨膜离子传输特性。然而,拓扑平凡的 Lehn 型环状六聚 FeII 螺旋结构(与溶液中的金属化大卫之星索烯具有相似的阴离子结合亲和力)也没有表现出离子传输特性。开环和闭环结构之间的行为差异可能是由连接基团的构象限制引起的,这可能增强了通道形成拓扑复杂分子的刚性。衍生自六聚环状螺旋的 (FeII)6 配位大卫星索烷在离子传输方面比衍生自相同环状五聚体的 (FeII)5 配位五叶结强 2 个数量级积木。功效降低让人想起用不正确的单体化学计量组装的多亚基蛋白质离子通道。











































 京公网安备 11010802027423号
京公网安备 11010802027423号