当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Carbon–Carbon Bond Cleavage of Cyclopropanols
Chemical Reviews ( IF 51.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.chemrev.0c00346 Tyler R. McDonald 1 , L. Reginald Mills 1 , Michael S. West 1 , Sophie A. L. Rousseaux 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2020-10-21 , DOI: 10.1021/acs.chemrev.0c00346 Tyler R. McDonald 1 , L. Reginald Mills 1 , Michael S. West 1 , Sophie A. L. Rousseaux 1
Affiliation
|
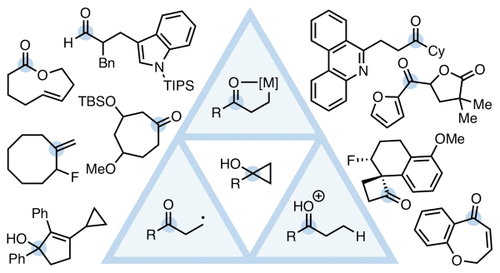
The carbon–carbon (C–C) bond cleavage of cyclopropanols is a wide area of research with much current activity. This review highlights new developments in this area over the past two decades. A summary is made of the three main reactivity modes, namely, homoenolate chemistry, β-keto radical chemistry, and acid-catalyzed ring-opening, as well as all other methods for the C–C bond cleavage and functionalization of cyclopropanols, including base-mediated ring-opening, metal-catalyzed C–C insertions and eliminations, oxidative fragmentation using hypervalent iodine reagents, reactions of donor–acceptor cyclopropanols, and pericylic reactions. Emphasis is placed on the synthetic utility of cyclopropanols and related derivatives, which have emerged as unique three-carbon synthons.
中文翻译:

环丙醇的选择性碳-碳键裂解
环丙醇的碳-碳(CC)键断裂是目前研究活跃的广泛领域。这篇评论重点介绍了过去二十年来该领域的新发展。总结了三种主要的反应模式,即均烯酸酯化学,β-酮自由基化学和酸催化的开环,以及所有其他用于环丙醇的CC键裂解和功能化的方法,包括碱介导的开环,金属催化的C–C插入和消除,使用高价碘试剂的氧化裂解,供体-受体环丙醇的反应以及周环反应。重点放在环丙醇和相关衍生物的合成用途上,它们已作为独特的三碳合成子出现。
更新日期:2020-10-21
中文翻译:

环丙醇的选择性碳-碳键裂解
环丙醇的碳-碳(CC)键断裂是目前研究活跃的广泛领域。这篇评论重点介绍了过去二十年来该领域的新发展。总结了三种主要的反应模式,即均烯酸酯化学,β-酮自由基化学和酸催化的开环,以及所有其他用于环丙醇的CC键裂解和功能化的方法,包括碱介导的开环,金属催化的C–C插入和消除,使用高价碘试剂的氧化裂解,供体-受体环丙醇的反应以及周环反应。重点放在环丙醇和相关衍生物的合成用途上,它们已作为独特的三碳合成子出现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号