当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Studies of the Mechanism of Carbamoylation of Nucleobases by Isocyanates
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.chemrestox.0c00220 Magnus Liljenberg 1 , Lena Ripa 2 , Igor Shamovsky 2
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.chemrestox.0c00220 Magnus Liljenberg 1 , Lena Ripa 2 , Igor Shamovsky 2
Affiliation
|
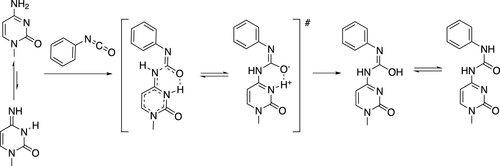
Isocyanates with the −N═C═O functional group are highly reactive compounds. They are used in various industrial applications and have been found as possible metabolites of hydroxamic acids. Isocyanates interact with biopolymers and are notorious mutagens. Mutagenic effects of isocyanates are caused by the formation of covalent adducts with nucleobases of DNA, primarily cytosines, through carbamoylation of NH2 groups to give the corresponding urea. The mechanism of carbamoylation of nucleobases by aryl isocyanates is studied by high-level density functional theory calculations. Three possible pathways are analyzed. It is demonstrated that the reaction follows the stepwise pathway, which starts with the formation of a π-complex followed by a rate-determining C–N covalent bond formation via the reactive tautomeric imine forms of the nucleobases. The reaction proceeds further through two consecutive proton transfers mediated by water molecules to give the final adduct. The predicted activation free energies of the rate-determining step in water agree with experimental data. In line with experiments, the reactivity of isocyanates toward nucleobases decreases in the order cytosine > adenine > guanine, and we rationalize this order of reactivity by the fall of their basicity and destabilization of the imine forms. Activation barriers of the alternative concerted pathways are higher than that of the preferred stepwise mechanism, and the match to experiment is poor. The kinetic effect of adding electron-withdrawing or electron-donating groups to the aryl group of aryl isocyanate is minute, which suggests that mutagenicity of isocyanates is determined exclusively by the reactivity of the −N═C═O group and as such cannot be removed by structural alterations of the adjacent aryl.
中文翻译:

异氰酸酯对核碱基氨基甲酰化机理的理论研究
具有-N=C=O 官能团的异氰酸酯是高反应性化合物。它们用于各种工业应用,并且已被发现是异羟肟酸的可能代谢物。异氰酸酯与生物聚合物相互作用,是臭名昭著的诱变剂。异氰酸酯的致突变作用是由通过 NH 2 的氨基甲酰化与 DNA 的核碱基(主要是胞嘧啶)形成共价加合物引起的组给予相应的尿素。通过高水平密度泛函理论计算研究了芳基异氰酸酯对核碱基氨基甲酰化的机制。分析了三种可能的途径。结果表明,该反应遵循逐步途径,首先形成 π 复合物,然后通过核碱基的反应性互变异构亚胺形式形成决定速率的 C-N 共价键。通过由水分子介导的两次连续质子转移,反应进一步进行,得到最终的加合物。水中决速步骤的预测活化自由能与实验数据一致。根据实验,异氰酸酯对核碱基的反应性以胞嘧啶 > 腺嘌呤 > 鸟嘌呤的顺序降低,我们通过亚胺形式的碱性下降和不稳定来使这种反应性顺序合理化。替代协同途径的激活障碍高于首选的逐步机制,与实验的匹配性较差。在异氰酸芳基酯的芳基上添加吸电子或给电子基团的动力学效应很小,这表明异氰酸酯的致突变性完全由-N=C=O基团的反应性决定,因此无法去除通过相邻芳基的结构改变。
更新日期:2020-11-16
中文翻译:

异氰酸酯对核碱基氨基甲酰化机理的理论研究
具有-N=C=O 官能团的异氰酸酯是高反应性化合物。它们用于各种工业应用,并且已被发现是异羟肟酸的可能代谢物。异氰酸酯与生物聚合物相互作用,是臭名昭著的诱变剂。异氰酸酯的致突变作用是由通过 NH 2 的氨基甲酰化与 DNA 的核碱基(主要是胞嘧啶)形成共价加合物引起的组给予相应的尿素。通过高水平密度泛函理论计算研究了芳基异氰酸酯对核碱基氨基甲酰化的机制。分析了三种可能的途径。结果表明,该反应遵循逐步途径,首先形成 π 复合物,然后通过核碱基的反应性互变异构亚胺形式形成决定速率的 C-N 共价键。通过由水分子介导的两次连续质子转移,反应进一步进行,得到最终的加合物。水中决速步骤的预测活化自由能与实验数据一致。根据实验,异氰酸酯对核碱基的反应性以胞嘧啶 > 腺嘌呤 > 鸟嘌呤的顺序降低,我们通过亚胺形式的碱性下降和不稳定来使这种反应性顺序合理化。替代协同途径的激活障碍高于首选的逐步机制,与实验的匹配性较差。在异氰酸芳基酯的芳基上添加吸电子或给电子基团的动力学效应很小,这表明异氰酸酯的致突变性完全由-N=C=O基团的反应性决定,因此无法去除通过相邻芳基的结构改变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号