当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Positively Charged C-Terminal Region of Human Skeletal Troponin T Retards Activation and Decreases Calcium Sensitivity
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.biochem.0c00499 Alfredo Jesus Lopez Davila 1 , Li Zhu 2 , Leon Fritz 1 , Theresia Kraft 1 , Joseph M. Chalovich 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.biochem.0c00499 Alfredo Jesus Lopez Davila 1 , Li Zhu 2 , Leon Fritz 1 , Theresia Kraft 1 , Joseph M. Chalovich 2
Affiliation
|
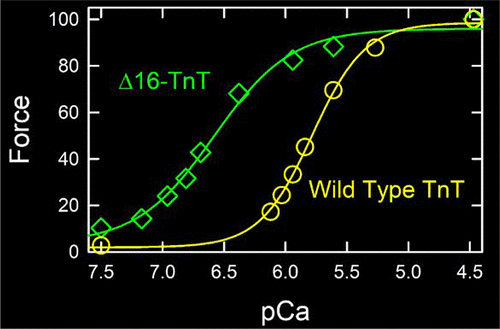
Calcium binding to troponin C (TnC) activates striated muscle contraction by removing TnI (troponin I) from its inhibitory site on actin. Troponin T (TnT) links TnI with tropomyosin, causing tropomyosin to move from an inhibitory position on actin to an activating position. Positive charges within the C-terminal region of human cardiac TnT limit Ca2+ activation. We now show that the positively charged region of TnT has an even larger impact on skeletal muscle regulation. We prepared one variant of human skeletal TnT that had the C-terminal 16 residues truncated (Δ16) and another with an added C-terminal Cys residue and Ala substituted for the last 6 basic residues (251C-HAHA). Both mutants reduced (based on S1 binding kinetics) or eliminated (based on acrylodan-tropomyosin fluorescence) the first inactive state of actin at <10 nM free Ca2+. 251C-HAHA-TnT and Δ16-TnT mutants greatly increased ATPase activation at 0.2 mM Ca2+, even without high-affinity cross-bridge binding. They also shifted the force–pCa curve of muscle fibers to lower Ca2+ by 0.8–1.2 pCa units (the larger shift for 251C-HAHA-TnT). Shifts in force–pCa were maintained in the presence of para-aminoblebbistatin. The effects of modification of the C-terminal region of TnT on the kinetics of S1 binding to actin were somewhat different from those observed earlier with the cardiac analogue. In general, the C-terminal region of human skeletal TnT is critical to regulation, just as it is in the cardiac system, and is a potential target for modulating activity.
中文翻译:

人骨骼肌钙蛋白T的正电荷C末端区域阻碍了激活并降低了钙敏感性
钙与肌钙蛋白C(TnC)的结合通过从肌动蛋白抑制位点去除TnI(肌钙蛋白I)激活横纹肌收缩。肌钙蛋白T(TnT)将TnI与原肌球蛋白连接,导致原肌球蛋白从肌动蛋白上的抑制位移动到激活位。人心脏TnT C端区域内的正电荷限制了Ca 2+激活。现在,我们显示TnT带正电的区域对骨骼肌调节的影响更大。我们制备了人骨骼TnT的一个变体,该变体的C末端16个残基被截短(Δ16),另一个变体具有添加的C末端Cys残基和Ala替代了最后6个碱性残基(251C-HAHA)。在<10 nM游离Ca 2+处,两个突变体均降低(基于S1结合动力学)或消除(基于丙烯酰原肌球蛋白荧光)肌动蛋白的第一个失活状态。251C-HAHA-TnT和Δ16-TnT突变体在没有高亲和力跨桥结合的情况下,在0.2 mM Ca 2+处极大地增加了ATPase的活化。他们还将肌肉纤维的力–pCa曲线移动到降低Ca 2+单位为0.8–1.2 pCa单位(对于251C-HAHA-TnT,位移较大)。在力-PCA的变化保持在存在对-aminoblebbistatin。TnT的C末端区域的修饰对S1与肌动蛋白结合动力学的影响与先前使用心脏类似物观察到的影响有些不同。通常,人体骨骼TnT的C末端区域对调节至关重要,就像在心脏系统中一样,并且是调节活性的潜在目标。
更新日期:2020-11-03
中文翻译:

人骨骼肌钙蛋白T的正电荷C末端区域阻碍了激活并降低了钙敏感性
钙与肌钙蛋白C(TnC)的结合通过从肌动蛋白抑制位点去除TnI(肌钙蛋白I)激活横纹肌收缩。肌钙蛋白T(TnT)将TnI与原肌球蛋白连接,导致原肌球蛋白从肌动蛋白上的抑制位移动到激活位。人心脏TnT C端区域内的正电荷限制了Ca 2+激活。现在,我们显示TnT带正电的区域对骨骼肌调节的影响更大。我们制备了人骨骼TnT的一个变体,该变体的C末端16个残基被截短(Δ16),另一个变体具有添加的C末端Cys残基和Ala替代了最后6个碱性残基(251C-HAHA)。在<10 nM游离Ca 2+处,两个突变体均降低(基于S1结合动力学)或消除(基于丙烯酰原肌球蛋白荧光)肌动蛋白的第一个失活状态。251C-HAHA-TnT和Δ16-TnT突变体在没有高亲和力跨桥结合的情况下,在0.2 mM Ca 2+处极大地增加了ATPase的活化。他们还将肌肉纤维的力–pCa曲线移动到降低Ca 2+单位为0.8–1.2 pCa单位(对于251C-HAHA-TnT,位移较大)。在力-PCA的变化保持在存在对-aminoblebbistatin。TnT的C末端区域的修饰对S1与肌动蛋白结合动力学的影响与先前使用心脏类似物观察到的影响有些不同。通常,人体骨骼TnT的C末端区域对调节至关重要,就像在心脏系统中一样,并且是调节活性的潜在目标。









































 京公网安备 11010802027423号
京公网安备 11010802027423号