当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Boosting the oxygen evolution activity in non-stoichiometric praseodymium ferrite-based perovskites by A site substitution for alkaline electrolyser anodes
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-10-20 , DOI: 10.1039/d0se01278e Steve Ward 1, 2, 3, 4 , Mark A. Isaacs 4, 5, 6, 7, 8 , Gaurav Gupta 1, 2, 3, 4, 9 , Mohamed Mamlouk 1, 2, 3, 4 , Stevin S. Pramana 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2020-10-20 , DOI: 10.1039/d0se01278e Steve Ward 1, 2, 3, 4 , Mark A. Isaacs 4, 5, 6, 7, 8 , Gaurav Gupta 1, 2, 3, 4, 9 , Mohamed Mamlouk 1, 2, 3, 4 , Stevin S. Pramana 1, 2, 3, 4
Affiliation
|
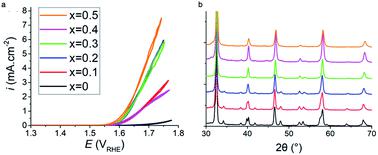
Sustainable fossil fuel free systems are crucial for tackling climate change in the global energy market, and the identification and understanding of catalysts needed to build these systems plays a vital role in their development. ABO3−δ perovskite oxides have been observed to be potential replacement materials for the high-performing, but low ionic conducting and economically unfavourable Pt and IrO2 water splitting catalysts. In this work increased addition of Sr2+ aliovalent dopant ions into the crystal lattice of Pr1−xSrxFeO3−δ perovskites via A site substitution was seen to drastically improve the electrocatalytic activity of the oxygen evolution reaction (OER) in alkaline environments. The undoped PrFeO3−δ catalyst was not catalytically active up to 1.70 V against the reversible hydrogen electrode (RHE), whilst an onset potential of 1.62 V was observed for x = 0.5. Increased strontium content in Pr1−xSrxFeO3−δ was found to cause a reduction in the lattice parameters and crystal volume whilst retaining the orthorhombic Pbnm space group throughout all dopant levels, analysed using the Rietveld method. However, it was noted that the orthorhombic distortion was reduced as more Sr2+ replaced Pr3+. The mechanism for the increased electrocatalytic activity with increased strontium is due to the increasing concentration of oxygen vacancy (δ), leading to increased catalyst site availability, and the increased average oxidation state of Fe cations, consistent with the iodometric titration results. This results in shifting the average d shell eg electron filling further towards unity. X-ray photoelectron spectrum of the O 1s core level also shows the presence of lattice oxide and surface hydroxide/carbonate. This work shows promise in that using the more abundant and more economically friendly material of strontium allows for improved OER catalytic activity in otherwise inactive perovskite catalyst oxides.
中文翻译:

通过碱性电解阳极的A位取代来提高非化学计量铁氧体基钙钛矿中氧的释放活性
可持续的不含化石燃料的系统对于应对全球能源市场中的气候变化至关重要,而识别和理解构建这些系统所需的催化剂对它们的发展至关重要。ABO 3- δ钙钛矿型氧化物已观察到是用于高性能,但低离子传导潜在的替代材料和经济上是不利的Pt和的IrO 2水分解催化剂。在这项工作中增加另外的Sr的2+异价掺杂剂离子进入的镨晶格1- X的Sr X的FeO 3- δ的钙钛矿通过在碱性环境中,观察到位取代可显着改善氧释放反应(OER)的电催化活性。未掺杂的PrFeO 3- δ催化剂不是催化活性高达针对可逆氢电极(RHE)1.70 V,同时观察到1.62 V的开始电位X = 0.5。在增加的锶含量镨1- X的Sr X的FeO 3- δ被发现导致晶格参数的降低和结晶体积,同时保持正交晶为Pbnm遍及所有掺杂剂水平空间群,利用Rietveld法进行分析。但是,应注意的是,随着Sr的增加,正交晶畸变会减小2+代替Pr 3+。与锶增加有关的电催化活性增加的机制是由于氧空位( δ)浓度的增加,导致催化剂位点可用性的增加,以及Fe阳离子平均氧化态的增加,这与碘量滴定法结果一致。这导致平均d壳e g电子填充进一步向统一移动。O 1s核能级的X射线光电子能谱还显示出晶格氧化物和表面氢氧化物/碳酸盐的存在。这项工作显示出希望,因为使用更丰富和更经济的锶材料可以提高原本非活性的钙钛矿型催化剂氧化物的OER催化活性。
更新日期:2020-12-17
中文翻译:

通过碱性电解阳极的A位取代来提高非化学计量铁氧体基钙钛矿中氧的释放活性
可持续的不含化石燃料的系统对于应对全球能源市场中的气候变化至关重要,而识别和理解构建这些系统所需的催化剂对它们的发展至关重要。ABO 3- δ钙钛矿型氧化物已观察到是用于高性能,但低离子传导潜在的替代材料和经济上是不利的Pt和的IrO 2水分解催化剂。在这项工作中增加另外的Sr的2+异价掺杂剂离子进入的镨晶格1- X的Sr X的FeO 3- δ的钙钛矿通过在碱性环境中,观察到位取代可显着改善氧释放反应(OER)的电催化活性。未掺杂的PrFeO 3- δ催化剂不是催化活性高达针对可逆氢电极(RHE)1.70 V,同时观察到1.62 V的开始电位X = 0.5。在增加的锶含量镨1- X的Sr X的FeO 3- δ被发现导致晶格参数的降低和结晶体积,同时保持正交晶为Pbnm遍及所有掺杂剂水平空间群,利用Rietveld法进行分析。但是,应注意的是,随着Sr的增加,正交晶畸变会减小2+代替Pr 3+。与锶增加有关的电催化活性增加的机制是由于氧空位( δ)浓度的增加,导致催化剂位点可用性的增加,以及Fe阳离子平均氧化态的增加,这与碘量滴定法结果一致。这导致平均d壳e g电子填充进一步向统一移动。O 1s核能级的X射线光电子能谱还显示出晶格氧化物和表面氢氧化物/碳酸盐的存在。这项工作显示出希望,因为使用更丰富和更经济的锶材料可以提高原本非活性的钙钛矿型催化剂氧化物的OER催化活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号