当前位置:
X-MOL 学术
›
Biopolymers
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expression and characterization of chimeric spidroins from flagelliform‐ aciniform repetitive modules
Biopolymers ( IF 3.2 ) Pub Date : 2020-10-19 , DOI: 10.1002/bip.23404 Lu-Yang Tian 1 , Qing Meng 1 , Ying Lin 1
Biopolymers ( IF 3.2 ) Pub Date : 2020-10-19 , DOI: 10.1002/bip.23404 Lu-Yang Tian 1 , Qing Meng 1 , Ying Lin 1
Affiliation
|
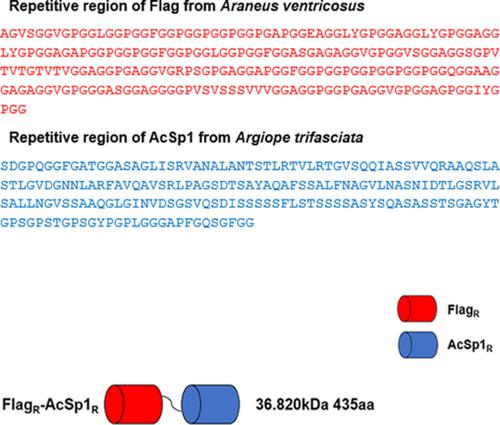
Spiders can produce up to seven different types of silks or glues with different mechanical properties. Of these, flagelliform (Flag) silk is the most elastic, and aciniform (AcSp1) silk is the toughest. To produce a chimeric spider silk (spidroin) FlagR‐AcSp1R, we fused one repetitive module of flagelliform silk from Araneus ventricosus and one repetitive module of aciniform silk from Argiope trifasciata. The recombinant protein expressed in E. coli formed silk‐like fibers by manual‐drawing. CD analysis showed that the secondary structure of FlagR‐AcSp1R spidroin remained stable during the gradual reduction of pH from 7.0 to 5.5. The spectrum of FTIR indicated that the secondary structure of FlagR‐AcSp1R changed from α‐helix to β‐sheet. The conformation change of FlagR‐AcSp1R was similar to other spidroins in the fiber formation process. SEM analysis revealed that the mean diameter of the fibers was around 1 ~ 2 μm, and the surface was smooth and uniform. The chimeric fibers exhibited superior toughness (~33.1 MJ/m3) and tensile strength (~261.4 MPa). This study provides new insight into design of chimeric spider silks with high mechanical properties.
中文翻译:

鞭毛状-腺泡状重复模块嵌合蜘蛛蛋白的表达和表征
蜘蛛可以生产多达七种不同类型的具有不同机械性能的丝或胶水。其中,鞭毛(Flag)丝弹性最强,腺泡(AcSp1)丝最坚韧。为了生产嵌合蜘蛛丝(蜘蛛蛋白)FlagR-AcSp1R,我们融合了来自腹壁蜘蛛的一个重复模块的鞭毛状丝和来自 Argiope trifasciata 的一个重复模块的腺泡状丝。在大肠杆菌中表达的重组蛋白通过手工拉伸形成丝状纤维。CD 分析表明,在 pH 从 7.0 逐渐降低到 5.5 的过程中,FlagR-AcSp1R spidroin 的二级结构保持稳定。FTIR光谱表明FlagR-AcSp1R的二级结构从α-螺旋变为β-折叠。在纤维形成过程中,FlagR-AcSp1R 的构象变化与其他蛛丝蛋白相似。SEM分析表明纤维的平均直径在1~2μm左右,表面光滑均匀。嵌合纤维表现出优异的韧性 (~33.1 MJ/m3) 和拉伸强度 (~261.4 MPa)。这项研究为设计具有高机械性能的嵌合蜘蛛丝提供了新的见解。
更新日期:2020-10-19
中文翻译:

鞭毛状-腺泡状重复模块嵌合蜘蛛蛋白的表达和表征
蜘蛛可以生产多达七种不同类型的具有不同机械性能的丝或胶水。其中,鞭毛(Flag)丝弹性最强,腺泡(AcSp1)丝最坚韧。为了生产嵌合蜘蛛丝(蜘蛛蛋白)FlagR-AcSp1R,我们融合了来自腹壁蜘蛛的一个重复模块的鞭毛状丝和来自 Argiope trifasciata 的一个重复模块的腺泡状丝。在大肠杆菌中表达的重组蛋白通过手工拉伸形成丝状纤维。CD 分析表明,在 pH 从 7.0 逐渐降低到 5.5 的过程中,FlagR-AcSp1R spidroin 的二级结构保持稳定。FTIR光谱表明FlagR-AcSp1R的二级结构从α-螺旋变为β-折叠。在纤维形成过程中,FlagR-AcSp1R 的构象变化与其他蛛丝蛋白相似。SEM分析表明纤维的平均直径在1~2μm左右,表面光滑均匀。嵌合纤维表现出优异的韧性 (~33.1 MJ/m3) 和拉伸强度 (~261.4 MPa)。这项研究为设计具有高机械性能的嵌合蜘蛛丝提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号