当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices
Aging Cell ( IF 8.0 ) Pub Date : 2020-10-20 , DOI: 10.1111/acel.13259 Farners Amargant 1 , Sharrón L Manuel 1 , Qing Tu 2 , Wendena S Parkes 3 , Felipe Rivas 4 , Luhan T Zhou 1 , Jennifer E Rowley 1 , Cecilia E Villanueva 3 , Jessica E Hornick 5 , Gajendra S Shekhawat 2 , Jian-Jun Wei 6 , Mary Ellen Pavone 1 , Adam R Hall 4 , Michele T Pritchard 3 , Francesca E Duncan 1
Aging Cell ( IF 8.0 ) Pub Date : 2020-10-20 , DOI: 10.1111/acel.13259 Farners Amargant 1 , Sharrón L Manuel 1 , Qing Tu 2 , Wendena S Parkes 3 , Felipe Rivas 4 , Luhan T Zhou 1 , Jennifer E Rowley 1 , Cecilia E Villanueva 3 , Jessica E Hornick 5 , Gajendra S Shekhawat 2 , Jian-Jun Wei 6 , Mary Ellen Pavone 1 , Adam R Hall 4 , Michele T Pritchard 3 , Francesca E Duncan 1
Affiliation
|
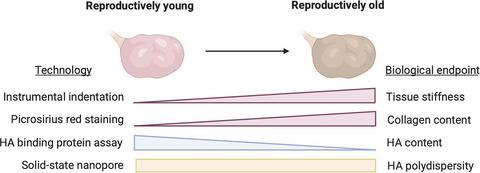
Fibrosis is a hallmark of aging tissues which often leads to altered architecture and function. The ovary is the first organ to show overt signs of aging, including increased fibrosis in the ovarian stroma. How this fibrosis affects ovarian biomechanics and the underlying mechanisms are unknown. Using instrumental indentation, we demonstrated a quantitative increase in ovarian stiffness, as evidenced by an increase in Young's modulus, when comparing ovaries from reproductively young (6–12 weeks) and old (14–17 months) mice. This ovarian stiffness was dependent on collagen because ex vivo enzyme‐mediated collagen depletion in ovaries from reproductively old mice restored their collagen content and biomechanical properties to those of young controls. In addition to collagen, we also investigated the role of hyaluronan (HA) in regulating ovarian stiffness. HA is an extracellular matrix glycosaminoglycan that maintains tissue homeostasis, and its loss can change the biomechanical properties of tissues. The total HA content in the ovarian stroma decreased with age, and this was associated with increased hyaluronidase (Hyal1) and decreased hyaluronan synthase (Has3) expression. These gene expression differences were not accompanied by changes in ovarian HA molecular mass distribution. Furthermore, ovaries from mice deficient in HAS3 were stiffer compared to age‐matched WT mice. Our results demonstrate that the ovary becomes stiffer with age and that both collagen and HA matrices are contributing mechanisms regulating ovarian biomechanics. Importantly, the age‐associated increase in collagen and decrease in HA are conserved in the human ovary and may impact follicle development and oocyte quality.
中文翻译:

哺乳动物卵巢的卵巢硬度随着年龄的增长而增加,并且取决于胶原蛋白和透明质酸基质
纤维化是老化组织的标志,通常会导致结构和功能改变。卵巢是第一个显示出明显衰老迹象的器官,包括卵巢间质纤维化增加。这种纤维化如何影响卵巢生物力学及其潜在机制尚不清楚。使用仪器压痕,我们证明了在比较年轻(6-12 周)和年老(14-17 个月)小鼠的卵巢时,卵巢硬度的定量增加,杨氏模量的增加证明了这一点。这种卵巢硬度依赖于胶原蛋白,因为离体酶介导的高龄小鼠卵巢中的胶原蛋白耗竭使它们的胶原蛋白含量和生物力学特性恢复到年轻对照组的水平。除了胶原蛋白,我们还研究了透明质酸 (HA) 在调节卵巢硬度方面的作用。HA 是一种维持组织稳态的细胞外基质糖胺聚糖,它的丢失会改变组织的生物力学特性。卵巢间质中的总 HA 含量随着年龄的增长而降低,这与透明质酸酶 (Hyal1) 增加和透明质酸合酶 ( Has3 ) 减少有关) 表达。这些基因表达差异不伴随卵巢 HA 分子量分布的变化。此外,与年龄匹配的 WT 小鼠相比,缺乏 HAS3 的小鼠的卵巢更硬。我们的结果表明,卵巢随着年龄的增长而变得更硬,并且胶原蛋白和 HA 基质都有助于调节卵巢生物力学的机制。重要的是,与年龄相关的胶原蛋白增加和 HA 减少在人类卵巢中是保守的,并且可能影响卵泡发育和卵母细胞质量。
更新日期:2020-11-23
中文翻译:

哺乳动物卵巢的卵巢硬度随着年龄的增长而增加,并且取决于胶原蛋白和透明质酸基质
纤维化是老化组织的标志,通常会导致结构和功能改变。卵巢是第一个显示出明显衰老迹象的器官,包括卵巢间质纤维化增加。这种纤维化如何影响卵巢生物力学及其潜在机制尚不清楚。使用仪器压痕,我们证明了在比较年轻(6-12 周)和年老(14-17 个月)小鼠的卵巢时,卵巢硬度的定量增加,杨氏模量的增加证明了这一点。这种卵巢硬度依赖于胶原蛋白,因为离体酶介导的高龄小鼠卵巢中的胶原蛋白耗竭使它们的胶原蛋白含量和生物力学特性恢复到年轻对照组的水平。除了胶原蛋白,我们还研究了透明质酸 (HA) 在调节卵巢硬度方面的作用。HA 是一种维持组织稳态的细胞外基质糖胺聚糖,它的丢失会改变组织的生物力学特性。卵巢间质中的总 HA 含量随着年龄的增长而降低,这与透明质酸酶 (Hyal1) 增加和透明质酸合酶 ( Has3 ) 减少有关) 表达。这些基因表达差异不伴随卵巢 HA 分子量分布的变化。此外,与年龄匹配的 WT 小鼠相比,缺乏 HAS3 的小鼠的卵巢更硬。我们的结果表明,卵巢随着年龄的增长而变得更硬,并且胶原蛋白和 HA 基质都有助于调节卵巢生物力学的机制。重要的是,与年龄相关的胶原蛋白增加和 HA 减少在人类卵巢中是保守的,并且可能影响卵泡发育和卵母细胞质量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号