当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
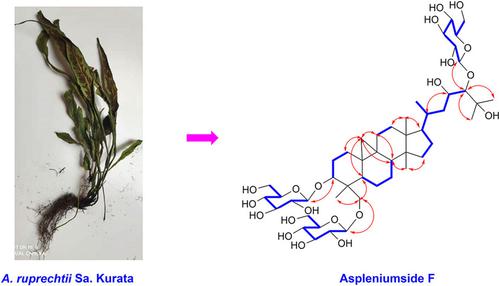
Structural Determination of A New Cycloartane Glycoside from Asplenium ruprechtii Sa. Kurata
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-11-09 , DOI: 10.1002/cbdv.202000500 Zhi-Bo Jiang 1 , Xiao-Xi Liu 2 , Jing-Zhi Chen 1 , Huan-Huan Guo 1 , Yun-Qi Hu 1 , Xin Guo 1 , Xiao-Li Ma 1 , Fang Wang 3 , Dai-Zhou Zhang 3 , Xiu-Li Wu 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-11-09 , DOI: 10.1002/cbdv.202000500 Zhi-Bo Jiang 1 , Xiao-Xi Liu 2 , Jing-Zhi Chen 1 , Huan-Huan Guo 1 , Yun-Qi Hu 1 , Xin Guo 1 , Xiao-Li Ma 1 , Fang Wang 3 , Dai-Zhou Zhang 3 , Xiu-Li Wu 2
Affiliation
|
We characterized a new cycloartane glycoside, herein known as aspleniumside F (1), along with five known compounds as kaempferol‐3‐O‐[(6‐O‐(E)‐feruloyl)‐β‐D‐glucopyranosyl]‐(1→2)‐β‐D‐galacopyranoside (2), quercetin‐3‐O‐[(6‐O‐(E)‐feruloyl)‐β‐D‐glucopyranosyl]‐(1→2)‐β‐D‐glucopyranoside (3), kaempferol‐3‐O‐[(6‐O‐(E)‐caffeoyl)‐β‐D‐glucopyranosyl]‐(1→2)‐β‐D‐glucopyranoside (4), kaempferol‐3‐O‐[(6‐O‐(E)‐caffeoyl)‐β‐D‐glucopyranosyl]‐(1→2)‐β‐D‐glucopyranosyl‐7‐O‐β‐D‐glucopyranoside (5), and kaempferol‐3‐O‐[(6‐O‐p‐coumaroyl)‐β‐D‐glucopyranosyl]‐(1→2)‐β‐D‐glucopyranosyl‐7‐O‐β‐D‐glucopyranoside (6), from Asplenium ruprechtii Sa. Kurata, a folk medicine widely used to treat Thromboangiitis obliterans in China, Japan, and Korea. Based on spectroscopic, mainly 1D‐, 2D‐NMR and (+)‐HR‐ESI‐MS, analyses as well as through comparisons with previous reports, its chemical structure was determined as 3β,24,30‐tri‐β‐D‐glucopyranosyl‐23,25‐dihydroxycycloartane (= (23R,24R)‐3β,24‐bis‐(β‐D‐glucopyranosyloxy)‐23,25‐dihydroxy‐9β‐9,19‐cyclolanostan‐29‐yl β‐D‐glucopyranoside). According to the 1H coupling constant of anomeric protons and co‐TLC of the acid hydrolysate with D‐glucose, all three glycoside groups in 1 were revealed as β‐D‐glucopyranosyl. Furthermore, SOD‐like antioxidant activity evaluation via IC50 of 12.43, 6.78, 9.12, 6.94 and 4.85 μM revealed that compounds 2–6 had bioactivity.
更新日期:2020-11-09











































 京公网安备 11010802027423号
京公网安备 11010802027423号