当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dithieno[a,e]pentalenes: Highly Antiaromatic Yet Stable π‐Electron Systems without Bulky Substituents
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-10-20 , DOI: 10.1002/chem.202004244 Junichi Usuba 1, 2 , Masahiro Hayakawa 1, 2 , Shigehiro Yamaguchi 2, 3 , Aiko Fukazawa 1, 3
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-10-20 , DOI: 10.1002/chem.202004244 Junichi Usuba 1, 2 , Masahiro Hayakawa 1, 2 , Shigehiro Yamaguchi 2, 3 , Aiko Fukazawa 1, 3
Affiliation
|
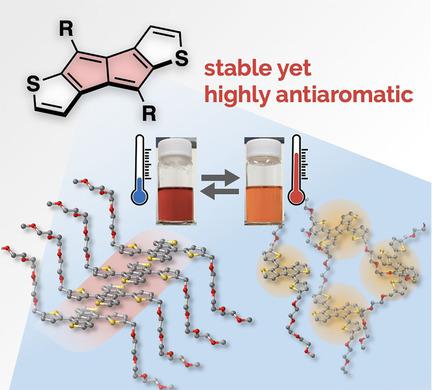
Dithieno[a,e]penalenes (DTPs) with various substituents were synthesized as a class of antiaromatic compounds. Annulation of thiophene rings imparts the pentalene moiety with high thermal stability even without bulky substituents, while retaining antiaromaticity. The higher magnitude of antiaromaticity in DTPs, in addition to the differences in the electronic structures of the fused aromatic rings, resulted in a narrower HOMO–LUMO gap than that of the corresponding dibenzo[a,e]pentalene analog, giving rise to red‐shifted electronic absorption that reaches the near‐infrared region. Moreover, systematic investigations on the solid‐state packing structure revealed that DTPs prefer offset face‐to‐face packing motifs rather than face‐centered π–π stacking. In particular, the thienyl‐substituted DTP bearing hydrophilic side chains exhibited thermochromic behavior in polar solvents, which was ascribed to the formation of aggregates.
中文翻译:

Dithieno [a,e] pentalenes:高度抗芳族但稳定的π电子体系,没有大的取代基
具有各种取代基的二噻吩并[ a,e ]对苯二酸酯(DTP)被合成为一类抗芳族化合物。噻吩环的环化赋予戊烯部分具有高的热稳定性,即使没有庞大的取代基,同时又保留了抗芳香性。除稠合芳环的电子结构不同外,DTP中较高的抗芳烃性导致其HOMO-LUMO间隙比相应的二苯并[ a,e]窄。戊烯类似物,引起红移电子吸收到达近红外区域。此外,对固态包装结构的系统研究表明,DTP更偏向于面对面的包装图案,而不是面向中心的π-π堆叠。特别是,带有噻吩基取代的DTP的亲水侧链在极性溶剂中表现出热致变色行为,这归因于聚集体的形成。
更新日期:2020-10-20
中文翻译:

Dithieno [a,e] pentalenes:高度抗芳族但稳定的π电子体系,没有大的取代基
具有各种取代基的二噻吩并[ a,e ]对苯二酸酯(DTP)被合成为一类抗芳族化合物。噻吩环的环化赋予戊烯部分具有高的热稳定性,即使没有庞大的取代基,同时又保留了抗芳香性。除稠合芳环的电子结构不同外,DTP中较高的抗芳烃性导致其HOMO-LUMO间隙比相应的二苯并[ a,e]窄。戊烯类似物,引起红移电子吸收到达近红外区域。此外,对固态包装结构的系统研究表明,DTP更偏向于面对面的包装图案,而不是面向中心的π-π堆叠。特别是,带有噻吩基取代的DTP的亲水侧链在极性溶剂中表现出热致变色行为,这归因于聚集体的形成。









































 京公网安备 11010802027423号
京公网安备 11010802027423号