当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Proton-Responsive Annulated Mesoionic Carbene (MIC) Scaffold on Ir Complex for Proton/Hydride Shuttle: An Experimental and Computational Investigation on Reductive Amination of Aldehyde
Organometallics ( IF 2.5 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.organomet.0c00568 Pragati Pandey 1 , Prosenjit Daw 1 , Noor U Din Reshi 1 , Kira R. Ehmann 2, 3 , Markus Hölscher 2 , Walter Leitner 2, 3 , Jitendra K. Bera 1
Organometallics ( IF 2.5 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.organomet.0c00568 Pragati Pandey 1 , Prosenjit Daw 1 , Noor U Din Reshi 1 , Kira R. Ehmann 2, 3 , Markus Hölscher 2 , Walter Leitner 2, 3 , Jitendra K. Bera 1
Affiliation
|
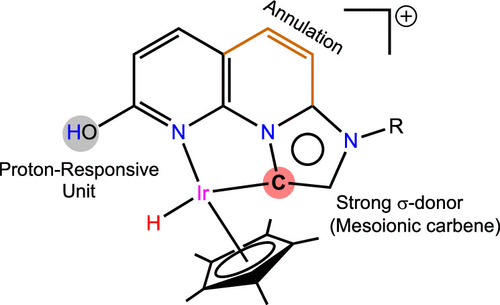
A Cp*Ir(III) complex (1) bearing a proton-responsive hydroxy unit on an annulated imidazo[1,2-a][1,8]naphthyridine based mesoionic carbene scaffold was synthesized by two different synthetic routes. The molecular structure of 1 revealed an anionic lactam form of the ligand. The acid–base equilibrium between the lactam-lactim tautomers on the ligand scaffold was examined by 1H NMR and UV–vis spectra. The pKa of the appendage −OH group in the lactim form of 1 was estimated to assess the proton transfer property of the catalyst. The catalytic efficacy of 1 for reductive amination of aldehyde was evaluated by utilizing three different hydrogen sources: molecular H2, iPrOH/KOtBu combination, and HCOOH/Et3N (5:2) azeotropic mixture. The HCOOH/Et3N (5:2) azeotropic mixture protocol was found to be the best among the three different hydrogenation methods. Catalyst 1 hydrogenates imines chemoselectively over carbonyls under the reaction conditions. A range of aldehydes was reductively aminated to the corresponding secondary amines using the HCOOH/Et3N (5:2) azeotropic mixture. Further, catalyst 1 showed high efficiency for the reduction of a wide variety of N-heterocyclic imine derivatives. The lactam-lactim tautomerization of the ligand system is proposed for direct hydrogenation, whereas only the lactam form operates in the strongly basic medium (iPrOH/KOtBu). Under HCOOH/Et3N (5:2) conditions, the lactam scaffold is not protonated; rather, an outer-sphere hydride transfer from formate to the Ir is proposed, which is supported by 1H NMR and DFT calculations. Finally, ligand-promoted hydride transfer from metal-hydride to the protonated imine affords the corresponding amine. A close agreement between the experimentally estimated and computed thermodynamic/kinetic parameters gives credence to the metal-ligand cooperative mechanism for the imine hydrogenation reaction using the HCOOH/Et3N (5:2) azeotropic mixture.
中文翻译:

用于质子/氢化物梭的Ir配合物的质子响应性环中离子碳(MIC)支架:醛还原胺化的实验和计算研究
通过两种不同的合成途径合成了在环状咪唑并[1,2- a ] [1,8]萘吡啶基介电卡宾骨架上带有质子响应性羟基单元的Cp * Ir(III)配合物(1)。的分子结构1揭示了配体的阴离子内酰胺形式。通过1 H NMR和UV-vis光谱检查了配体支架上内酰胺-内酯互变异构体之间的酸碱平衡。估算内酰胺形式为1的附件-OH基团的p K a,以评估催化剂的质子转移性能。1的催化功效通过使用三种不同的氢源(分子H 2,i PrOH / KO t Bu组合和HCOOH / Et 3 N(5:2)共沸混合物)评估醛的还原胺化反应。发现在三种不同的氢化方法中,HCOOH / Et 3 N(5:2)共沸混合物是最好的。在反应条件下,催化剂1在羰基上化学选择性地氢化亚胺。使用HCOOH / Et 3 N(5:2)共沸混合物将一系列醛还原为相应的仲胺。此外,催化剂1在还原多种N-杂环亚胺衍生物方面显示出高效率。提议将配体系统的内酰胺-内酰胺互变异构体用于直接氢化,而仅内酰胺形式在强碱性介质(i PrOH / KO t Bu)中起作用。在HCOOH / Et 3 N(5:2)条件下,内酰胺支架不被质子化。而是提出了从甲酸盐到Ir的外球氢化物转移,这受11 H NMR和DFT计算。最后,配体促进的氢化物从金属氢化物转移到质子化的亚胺,得到相应的胺。实验估计和计算的热力学/动力学参数之间的密切一致性为使用HCOOH / Et 3 N(5:2)共沸混合物的亚胺加氢反应的金属-配体协同机理提供了依据。
更新日期:2020-11-09
中文翻译:

用于质子/氢化物梭的Ir配合物的质子响应性环中离子碳(MIC)支架:醛还原胺化的实验和计算研究
通过两种不同的合成途径合成了在环状咪唑并[1,2- a ] [1,8]萘吡啶基介电卡宾骨架上带有质子响应性羟基单元的Cp * Ir(III)配合物(1)。的分子结构1揭示了配体的阴离子内酰胺形式。通过1 H NMR和UV-vis光谱检查了配体支架上内酰胺-内酯互变异构体之间的酸碱平衡。估算内酰胺形式为1的附件-OH基团的p K a,以评估催化剂的质子转移性能。1的催化功效通过使用三种不同的氢源(分子H 2,i PrOH / KO t Bu组合和HCOOH / Et 3 N(5:2)共沸混合物)评估醛的还原胺化反应。发现在三种不同的氢化方法中,HCOOH / Et 3 N(5:2)共沸混合物是最好的。在反应条件下,催化剂1在羰基上化学选择性地氢化亚胺。使用HCOOH / Et 3 N(5:2)共沸混合物将一系列醛还原为相应的仲胺。此外,催化剂1在还原多种N-杂环亚胺衍生物方面显示出高效率。提议将配体系统的内酰胺-内酰胺互变异构体用于直接氢化,而仅内酰胺形式在强碱性介质(i PrOH / KO t Bu)中起作用。在HCOOH / Et 3 N(5:2)条件下,内酰胺支架不被质子化。而是提出了从甲酸盐到Ir的外球氢化物转移,这受11 H NMR和DFT计算。最后,配体促进的氢化物从金属氢化物转移到质子化的亚胺,得到相应的胺。实验估计和计算的热力学/动力学参数之间的密切一致性为使用HCOOH / Et 3 N(5:2)共沸混合物的亚胺加氢反应的金属-配体协同机理提供了依据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号