当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Bispidine-Based Chiral Amine Catalyst for Asymmetric Mannich Reaction of Ketones with Isatin Ketimines
Organic Letters ( IF 4.9 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.orglett.0c03305 Gonglin Li 1 , Mohuizi Liu 1 , Sijia Zou 1 , Xiaoming Feng 1 , Lili Lin 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.orglett.0c03305 Gonglin Li 1 , Mohuizi Liu 1 , Sijia Zou 1 , Xiaoming Feng 1 , Lili Lin 1
Affiliation
|
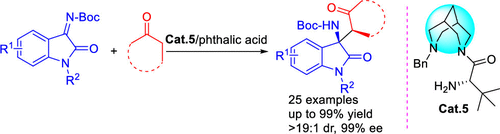
A unique chiral amine organocatalyst with a bispidine structure was found to be efficient for the diastereo- and enantioselective Mannich reaction of isatin ketimines with ketones. A series of 3-substituted 3-amino-2-oxindoles bearing vicinal tertiary and quaternary chiral stereogenic centers were obtained in excellent yields with excellent dr and ee values. The gram-scale synthesis and transformation of the product showed the practicability of this methodology. In addition, a possible transition state model was proposed to explain the origin of the stereoselectivity.
中文翻译:

双联吡啶为基础的手性胺催化剂,用于酮与Isatin Ketimines的不对称Mannich反应
发现具有联吡啶结构的独特手性胺有机催化剂可有效地用于伊斯兰酮酮与酮的非对映和对映选择性曼尼希反应。以优异的收率和优异的dr和ee值获得了一系列带有邻位叔和手性立体中心的3-取代的3-氨基-2-氧吲哚。产物的克级合成和转化表明了该方法的实用性。另外,提出了一种可能的过渡态模型来解释立体选择性的起源。
更新日期:2020-11-06
中文翻译:

双联吡啶为基础的手性胺催化剂,用于酮与Isatin Ketimines的不对称Mannich反应
发现具有联吡啶结构的独特手性胺有机催化剂可有效地用于伊斯兰酮酮与酮的非对映和对映选择性曼尼希反应。以优异的收率和优异的dr和ee值获得了一系列带有邻位叔和手性立体中心的3-取代的3-氨基-2-氧吲哚。产物的克级合成和转化表明了该方法的实用性。另外,提出了一种可能的过渡态模型来解释立体选择性的起源。










































 京公网安备 11010802027423号
京公网安备 11010802027423号