当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenative Metathesis of Enynes via Piano-Stool Ruthenium Carbene Complexes Formed by Alkyne gem-Hydrogenation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-19 , DOI: 10.1021/jacs.0c07808 Sebastian Peil 1 , Giovanni Bistoni 1 , Richard Goddard 1 , Alois Fürstner 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-19 , DOI: 10.1021/jacs.0c07808 Sebastian Peil 1 , Giovanni Bistoni 1 , Richard Goddard 1 , Alois Fürstner 1
Affiliation
|
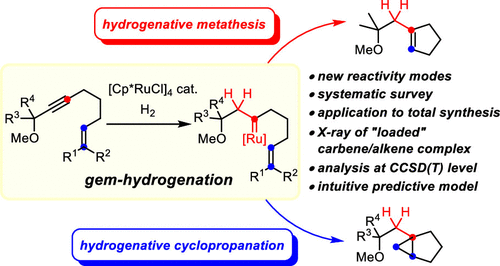
The only recently discovered gem-hydrogenation of internal alkynes is a fundamentally new transformation, in which both H atoms of dihydrogen are transferred to the same C atom of a triple bond while the other position transforms into a discrete metal carbene complex. [Cp*RuCl]4 is presently the catalyst of choice: the resulting piano-stool ruthenium carbenes can engage a tethered alkene into either cyclopropanation or metathesis, and a prototypical example of such a reactive intermediate with an olefin ligated to the ruthenium center has been isolated and characterized by X-ray diffraction. It is the substitution pattern of the olefin that determines whether metathesis or cyclopropanation takes place: a systematic survey using alkenes of largely different character in combination with a computational study of the mechanism at the local coupled cluster level of theory allowed the preparative results to be sorted and an intuitive model with predictive power to be proposed. This model links the course of the reaction to the polarization of the double bond as well as to the stability of the secondary carbene complex formed, if metathesis were to take place. The first application of “hydrogenative metathesis” to the total synthesis of sinularones E and F concurred with this interpretation and allowed the proposed structure of these marine natural products to be confirmed. During this synthesis, it was found that gem-hydrogenation also provides opportunities for C–H functionalization. Moreover, silylated alkynes are shown to participate well in hydrogenative metathesis, which opens a new entry into valuable allylsilane building blocks. Crystallographic evidence suggests that the polarized [Ru–Cl] bond of the catalyst interacts with the neighboring R3Si group. Since attractive interligand Cl/R3Si contacts had already previously been invoked to explain the outcome of various ruthenium-catalyzed reactions, including trans-hydrosilylation, the experimental confirmation provided herein has implications beyond the present case.
中文翻译:

通过炔烃宝石氢化形成的钢琴凳钌卡宾配合物进行烯炔的氢化复分解
最近发现的内部炔烃的宝石氢化是一种全新的转变,其中二氢的两个 H 原子转移到三键的同一个 C 原子上,而另一个位置转变为离散的金属卡宾络合物。 [Cp*RuCl]4 目前是首选的催化剂:所得的钢琴凳钌卡宾可以使束缚烯烃参与环丙烷化或复分解,并且这种具有连接到钌中心的烯烃的反应中间体的典型例子是分离并通过 X 射线衍射表征。烯烃的取代模式决定了是否发生复分解或环丙烷化:使用性质差异很大的烯烃进行的系统调查结合局部耦合簇理论水平的机理计算研究,可以对制备结果进行排序以及提出一个具有预测能力的直观模型。该模型将反应过程与双键的极化以及形成的二级卡宾配合物的稳定性(如果发生复分解)联系起来。 “氢化复分解”在 Sinularones E 和 F 的全合成中的首次应用与这种解释一致,并使得这些海洋天然产物的拟议结构得到了证实。在此合成过程中,我们发现宝石氢化也为 C-H 官能化提供了机会。此外,硅烷化炔烃被证明能够很好地参与氢化复分解反应,这为有价值的烯丙基硅烷结构单元开辟了新的途径。晶体学证据表明催化剂的极化[Ru-Cl]键与邻近的R3Si基团相互作用。 由于有吸引力的配体间 Cl/R3Si 接触先前已被用来解释各种钌催化反应(包括反式氢化硅烷化)的结果,因此本文提供的实验确认具有超出当前情况的含义。
更新日期:2020-10-19
中文翻译:

通过炔烃宝石氢化形成的钢琴凳钌卡宾配合物进行烯炔的氢化复分解
最近发现的内部炔烃的宝石氢化是一种全新的转变,其中二氢的两个 H 原子转移到三键的同一个 C 原子上,而另一个位置转变为离散的金属卡宾络合物。 [Cp*RuCl]4 目前是首选的催化剂:所得的钢琴凳钌卡宾可以使束缚烯烃参与环丙烷化或复分解,并且这种具有连接到钌中心的烯烃的反应中间体的典型例子是分离并通过 X 射线衍射表征。烯烃的取代模式决定了是否发生复分解或环丙烷化:使用性质差异很大的烯烃进行的系统调查结合局部耦合簇理论水平的机理计算研究,可以对制备结果进行排序以及提出一个具有预测能力的直观模型。该模型将反应过程与双键的极化以及形成的二级卡宾配合物的稳定性(如果发生复分解)联系起来。 “氢化复分解”在 Sinularones E 和 F 的全合成中的首次应用与这种解释一致,并使得这些海洋天然产物的拟议结构得到了证实。在此合成过程中,我们发现宝石氢化也为 C-H 官能化提供了机会。此外,硅烷化炔烃被证明能够很好地参与氢化复分解反应,这为有价值的烯丙基硅烷结构单元开辟了新的途径。晶体学证据表明催化剂的极化[Ru-Cl]键与邻近的R3Si基团相互作用。 由于有吸引力的配体间 Cl/R3Si 接触先前已被用来解释各种钌催化反应(包括反式氢化硅烷化)的结果,因此本文提供的实验确认具有超出当前情况的含义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号