当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Ring-Closing Reaction via C–N Bond Metathesis for Rapid Construction of Saturated N-Heterocycles
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-19 , DOI: 10.1021/jacs.0c10615 Bangkui Yu 1 , Suchen Zou 1 , Hongchi Liu 1 , Hanmin Huang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-19 , DOI: 10.1021/jacs.0c10615 Bangkui Yu 1 , Suchen Zou 1 , Hongchi Liu 1 , Hanmin Huang 1, 2
Affiliation
|
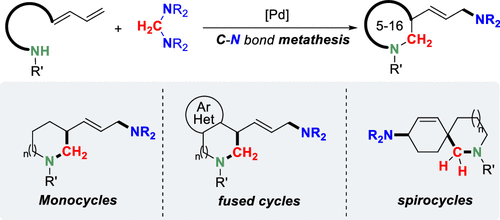
The ring-closing reactions based on chemical bond metathesis enable the efficient construction of a wide variety of cyclic systems which receive broad interest from medicinal and organic communities. However, the analogous reaction with C-N bond metathesis as a strategic fundamental step remains an unanswered challenge. Herein, we report the design of a new fundamental metallic C-N bond metathesis reaction that enables the palladium-catalyzed ring-closing reaction of aminodienes with aminals. The reactions proceed efficiently under mild conditions and exhibit broad substrate generality and functional group compatibility, leading to a wide variety of 5- to 16-membered N-heterocycles bearing diverse frameworks and functional groups.
中文翻译:

通过 C-N 键复分解钯催化的闭环反应快速构建饱和 N-杂环
基于化学键复分解的闭环反应能够有效构建各种循环系统,这些系统受到医学和有机界的广泛关注。然而,将 CN 键复分解作为战略性基本步骤的类似反应仍然是一个悬而未决的挑战。在此,我们报告了一种新的基本金属 CN 键复分解反应的设计,该反应能够实现钯催化的氨基二烯与缩醛胺的闭环反应。该反应在温和的条件下有效进行,并表现出广泛的底物通用性和官能团兼容性,从而产生各种具有不同骨架和官能团的 5 至 16 元 N-杂环。
更新日期:2020-10-19
中文翻译:

通过 C-N 键复分解钯催化的闭环反应快速构建饱和 N-杂环
基于化学键复分解的闭环反应能够有效构建各种循环系统,这些系统受到医学和有机界的广泛关注。然而,将 CN 键复分解作为战略性基本步骤的类似反应仍然是一个悬而未决的挑战。在此,我们报告了一种新的基本金属 CN 键复分解反应的设计,该反应能够实现钯催化的氨基二烯与缩醛胺的闭环反应。该反应在温和的条件下有效进行,并表现出广泛的底物通用性和官能团兼容性,从而产生各种具有不同骨架和官能团的 5 至 16 元 N-杂环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号