当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PD-L1 Glycosylation and Its Impact on Binding to Clinical Antibodies
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.jproteome.0c00521 Julius Benicky 1, 2 , Miloslav Sanda 1, 2 , Zuzana Brnakova Kennedy 1, 2 , Oliver C Grant 3 , Robert J Woods 3 , Alan Zwart 1 , Radoslav Goldman 1, 2, 4
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2020-10-19 , DOI: 10.1021/acs.jproteome.0c00521 Julius Benicky 1, 2 , Miloslav Sanda 1, 2 , Zuzana Brnakova Kennedy 1, 2 , Oliver C Grant 3 , Robert J Woods 3 , Alan Zwart 1 , Radoslav Goldman 1, 2, 4
Affiliation
|
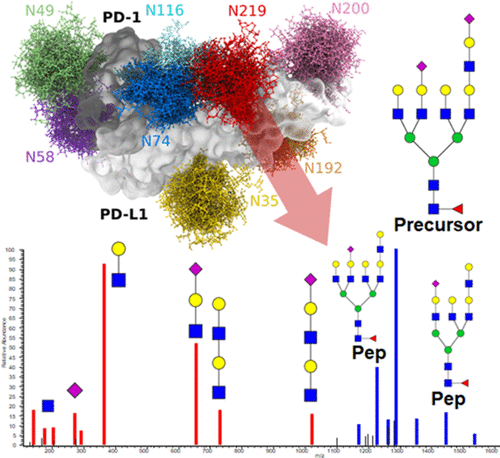
Immune checkpoint inhibitors, including PD-L1/PD-1, are key regulators of the immune response and promising targets in cancer immunotherapy. N-glycosylation of PD-L1 affects its interaction with PD-1, but little is known about the distribution of glycoforms at its four NXS/T sequons. We optimized LC–MS/MS methods using collision energy modulation for the site-specific resolution of specific glycan motifs. We demonstrate that PD-L1 on the surface of breast cancer cell line carries mostly complex glycans with a high proportion of polyLacNAc structures at the N219 sequon. Contrary to the full-length protein, the secreted form of PD-L1 expressed in breast MDA-MB-231 or HEK293 cells demonstrated minimum N219 occupancy and low contribution of the polyLacNAc structures. Molecular modeling of PD-L1/PD-1 interaction with N-glycans suggests that glycans at the N219 site of PD-L1 and N74 and N116 of PD-1 may be involved in glycan–glycan interactions, but the impact of this potential interaction on the protein function remains at this point unknown. The interaction of PD-L1 with clinical antibodies is also affected by glycosylation. In conclusion, PD-L1 expressed in the MDA-MB-231 breast cancer cell line carries polyLacNAc glycans mostly at the N219 sequon, which displays the highest variability in occupancy and is most likely to influence the interaction with PD-1.
中文翻译:

PD-L1 糖基化及其对临床抗体结合的影响
免疫检查点抑制剂,包括 PD-L1/PD-1,是免疫反应的关键调节剂和癌症免疫治疗的有希望的靶点。PD-L1 的 N-糖基化影响其与 PD-1 的相互作用,但对其四个 NXS/T 序列的糖型分布知之甚少。我们使用碰撞能量调制优化了 LC-MS/MS 方法,以实现特定聚糖基序的位点特异性分辨率。我们证明乳腺癌细胞系表面上的 PD-L1 主要携带复杂的聚糖,在 N219 序列中具有高比例的 polyLacNAc 结构。与全长蛋白相反,在乳腺 MDA-MB-231 或 HEK293 细胞中表达的 PD-L1 的分泌形式表现出最低的 N219 占有率和聚LacNAc 结构的低贡献。PD-L1/PD-1 与 N-聚糖相互作用的分子模型表明 PD-L1 的 N219 位点和 PD-1 的 N74 和 N116 处的聚糖可能参与聚糖-聚糖相互作用,但这种潜在相互作用的影响关于蛋白质的功能在这一点上仍然未知。PD-L1 与临床抗体的相互作用也受糖基化的影响。总之,在 MDA-MB-231 乳腺癌细胞系中表达的 PD-L1 主要在 N219 序列中携带 polyLacNAc 聚糖,该序列在占用率方面表现出最高的可变性,并且最有可能影响与 PD-1 的相互作用。PD-L1 与临床抗体的相互作用也受糖基化的影响。总之,在 MDA-MB-231 乳腺癌细胞系中表达的 PD-L1 主要在 N219 序列中携带 polyLacNAc 聚糖,该序列在占用率方面表现出最高的可变性,并且最有可能影响与 PD-1 的相互作用。PD-L1 与临床抗体的相互作用也受糖基化的影响。总之,在 MDA-MB-231 乳腺癌细胞系中表达的 PD-L1 主要在 N219 序列中携带 polyLacNAc 聚糖,该序列在占用率方面表现出最高的可变性,并且最有可能影响与 PD-1 的相互作用。
更新日期:2020-10-19
中文翻译:

PD-L1 糖基化及其对临床抗体结合的影响
免疫检查点抑制剂,包括 PD-L1/PD-1,是免疫反应的关键调节剂和癌症免疫治疗的有希望的靶点。PD-L1 的 N-糖基化影响其与 PD-1 的相互作用,但对其四个 NXS/T 序列的糖型分布知之甚少。我们使用碰撞能量调制优化了 LC-MS/MS 方法,以实现特定聚糖基序的位点特异性分辨率。我们证明乳腺癌细胞系表面上的 PD-L1 主要携带复杂的聚糖,在 N219 序列中具有高比例的 polyLacNAc 结构。与全长蛋白相反,在乳腺 MDA-MB-231 或 HEK293 细胞中表达的 PD-L1 的分泌形式表现出最低的 N219 占有率和聚LacNAc 结构的低贡献。PD-L1/PD-1 与 N-聚糖相互作用的分子模型表明 PD-L1 的 N219 位点和 PD-1 的 N74 和 N116 处的聚糖可能参与聚糖-聚糖相互作用,但这种潜在相互作用的影响关于蛋白质的功能在这一点上仍然未知。PD-L1 与临床抗体的相互作用也受糖基化的影响。总之,在 MDA-MB-231 乳腺癌细胞系中表达的 PD-L1 主要在 N219 序列中携带 polyLacNAc 聚糖,该序列在占用率方面表现出最高的可变性,并且最有可能影响与 PD-1 的相互作用。PD-L1 与临床抗体的相互作用也受糖基化的影响。总之,在 MDA-MB-231 乳腺癌细胞系中表达的 PD-L1 主要在 N219 序列中携带 polyLacNAc 聚糖,该序列在占用率方面表现出最高的可变性,并且最有可能影响与 PD-1 的相互作用。PD-L1 与临床抗体的相互作用也受糖基化的影响。总之,在 MDA-MB-231 乳腺癌细胞系中表达的 PD-L1 主要在 N219 序列中携带 polyLacNAc 聚糖,该序列在占用率方面表现出最高的可变性,并且最有可能影响与 PD-1 的相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号