当前位置:
X-MOL 学术
›
J. Leukoc. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peripheral leptin signaling persists in innate immune cells during diet-induced obesity
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2020-10-18 , DOI: 10.1002/jlb.3ab0820-092rr Glaucia Souza-Almeida 1, 2 , Lohanna Palhinha 1 , Sally Liechocki 1 , Jéssica Aparecida da Silva Pereira 1 , Patrícia Alves Reis 1 , Paula Ribeiro Braga Dib 3, 4 , Eugenio D Hottz 1, 3 , Jacy Gameiro 4 , Adriana Lima Vallochi 1 , Cecília Jacques de Almeida 1 , Hugo Castro-Faria-Neto 1 , Patrícia T Bozza 1 , Clarissa Menezes Maya-Monteiro 1
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2020-10-18 , DOI: 10.1002/jlb.3ab0820-092rr Glaucia Souza-Almeida 1, 2 , Lohanna Palhinha 1 , Sally Liechocki 1 , Jéssica Aparecida da Silva Pereira 1 , Patrícia Alves Reis 1 , Paula Ribeiro Braga Dib 3, 4 , Eugenio D Hottz 1, 3 , Jacy Gameiro 4 , Adriana Lima Vallochi 1 , Cecília Jacques de Almeida 1 , Hugo Castro-Faria-Neto 1 , Patrícia T Bozza 1 , Clarissa Menezes Maya-Monteiro 1
Affiliation
|
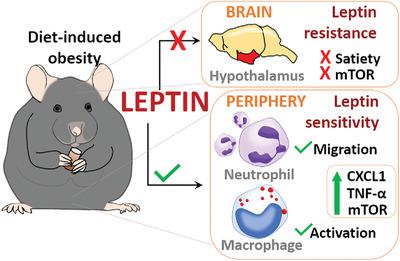
Leptin is a pleiotropic adipokine that regulates immunometabolism centrally and peripherally. Obese individuals present increased levels of leptin in the blood and develop hypothalamic resistance to this adipokine. Here we investigated whether leptin effects on the periphery are maintained despite the hypothalamic resistance. We previously reported that leptin injection induces in vivo neutrophil migration and peritoneal macrophage activation in lean mice through TNF-α- and CXCL1-dependent mechanisms. However, leptin effects on leukocyte biology during obesity remain unclear. In this study, we investigated the in vivo responsiveness of leukocytes to i.p. injected leptin in mice with diet-induced obesity (DIO). After 14–16 wk, high-sucrose, high-fat diet (HFD)-fed mice showed hyperglycemia, hyperleptinemia, and dyslipidemia compared to normal-sucrose, normal-fat diet (ND). Exogenous leptin did not reduce food intake in DIO mice in contrast to control mice, indicating that DIO mice were centrally resistant to leptin. Regardless of the diet, we found increased levels of TNF-α and CXCL1 in the animals injected with leptin, alongside a pronounced neutrophil migration to the peritoneal cavity and enhanced biogenesis of lipid droplets in peritoneal macrophages. Supporting our in vivo results, data from ex vivo leptin stimulation experiments confirmed hypothalamic resistance in DIO mice, whereas bone marrow cells responded to leptin stimulation through mTOR signaling despite obesity. Altogether, our results show that leukocytes responded equally to leptin in ND- or HFD-fed mice. These results support a role for leptin in the innate immune response also in obesity, contributing to the inflammatory status that leads to the development of metabolic disease.
中文翻译:

在饮食诱导的肥胖期间,外周瘦素信号在先天免疫细胞中持续存在
瘦素是一种多效性脂肪因子,可在中枢和外周调节免疫代谢。肥胖个体血液中的瘦素水平升高,并对这种脂肪因子产生下丘脑抵抗。在这里,我们研究了尽管存在下丘脑抵抗,但瘦素对外周的影响是否仍然存在。我们之前报道过瘦素注射通过 TNF-α 和 CXCL1 依赖性机制诱导瘦小鼠体内中性粒细胞迁移和腹腔巨噬细胞活化。然而,肥胖期间瘦素对白细胞生物学的影响仍不清楚。在这项研究中,我们研究了白细胞对饮食诱导肥胖 (DIO) 小鼠腹腔注射瘦素的体内反应性。14-16 周后,高蔗糖、高脂肪饮食 (HFD) 喂养的小鼠出现高血糖、高瘦素血症、和血脂异常与正常蔗糖、正常脂肪饮食 (ND) 相比。与对照小鼠相比,外源性瘦素并未减少 DIO 小鼠的食物摄入量,表明 DIO 小鼠对瘦素具有中枢抗性。无论饮食如何,我们发现注射瘦素的动物体内 TNF-α 和 CXCL1 的水平增加,同时中性粒细胞明显迁移到腹腔,并增强了腹腔巨噬细胞中脂滴的生物合成。支持我们的体内结果,来自体外瘦素刺激实验的数据证实了 DIO 小鼠的下丘脑抵抗,而尽管肥胖,骨髓细胞通过 mTOR 信号传导对瘦素刺激作出反应。总而言之,我们的结果表明,白细胞对 ND 或 HFD 喂养的小鼠中的瘦素反应相同。
更新日期:2020-10-18
中文翻译:

在饮食诱导的肥胖期间,外周瘦素信号在先天免疫细胞中持续存在
瘦素是一种多效性脂肪因子,可在中枢和外周调节免疫代谢。肥胖个体血液中的瘦素水平升高,并对这种脂肪因子产生下丘脑抵抗。在这里,我们研究了尽管存在下丘脑抵抗,但瘦素对外周的影响是否仍然存在。我们之前报道过瘦素注射通过 TNF-α 和 CXCL1 依赖性机制诱导瘦小鼠体内中性粒细胞迁移和腹腔巨噬细胞活化。然而,肥胖期间瘦素对白细胞生物学的影响仍不清楚。在这项研究中,我们研究了白细胞对饮食诱导肥胖 (DIO) 小鼠腹腔注射瘦素的体内反应性。14-16 周后,高蔗糖、高脂肪饮食 (HFD) 喂养的小鼠出现高血糖、高瘦素血症、和血脂异常与正常蔗糖、正常脂肪饮食 (ND) 相比。与对照小鼠相比,外源性瘦素并未减少 DIO 小鼠的食物摄入量,表明 DIO 小鼠对瘦素具有中枢抗性。无论饮食如何,我们发现注射瘦素的动物体内 TNF-α 和 CXCL1 的水平增加,同时中性粒细胞明显迁移到腹腔,并增强了腹腔巨噬细胞中脂滴的生物合成。支持我们的体内结果,来自体外瘦素刺激实验的数据证实了 DIO 小鼠的下丘脑抵抗,而尽管肥胖,骨髓细胞通过 mTOR 信号传导对瘦素刺激作出反应。总而言之,我们的结果表明,白细胞对 ND 或 HFD 喂养的小鼠中的瘦素反应相同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号