当前位置:
X-MOL 学术
›
J. Leukoc. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypoxia-inducible factors not only regulate but also are myeloid-cell treatment targets
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2020-10-18 , DOI: 10.1002/jlb.4ri0820-535r Lovis Kling 1 , Adrian Schreiber 1, 2 , Kai-Uwe Eckardt 1 , Ralph Kettritz 1, 2
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2020-10-18 , DOI: 10.1002/jlb.4ri0820-535r Lovis Kling 1 , Adrian Schreiber 1, 2 , Kai-Uwe Eckardt 1 , Ralph Kettritz 1, 2
Affiliation
|
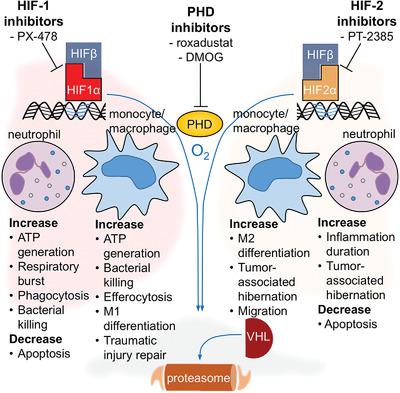
Hypoxia describes limited oxygen availability at the cellular level. Myeloid cells are exposed to hypoxia at various bodily sites and even contribute to hypoxia by consuming large amounts of oxygen during respiratory burst. Hypoxia-inducible factors (HIFs) are ubiquitously expressed heterodimeric transcription factors, composed of an oxygen-dependent α and a constitutive β subunit. The stability of HIF-1α and HIF-2α is regulated by oxygen-sensing prolyl-hydroxylases (PHD). HIF-1α and HIF-2α modify the innate immune response and are context dependent. We provide a historic perspective of HIF discovery, discuss the molecular components of the HIF pathway, and how HIF-dependent mechanisms modify myeloid cell functions. HIFs enable myeloid-cell adaptation to hypoxia by up-regulating anaerobic glycolysis. In addition to effects on metabolism, HIFs control chemotaxis, phagocytosis, degranulation, oxidative burst, and apoptosis. HIF-1α enables efficient infection defense by myeloid cells. HIF-2α delays inflammation resolution and decreases antitumor effects by promoting tumor-associated myeloid-cell hibernation. PHDs not only control HIF degradation, but also regulate the crosstalk between innate and adaptive immune cells thereby suppressing autoimmunity. HIF-modifying pharmacologic compounds are entering clinical practice. Current indications include renal anemia and certain cancers. Beneficial and adverse effects on myeloid cells should be considered and could possibly lead to drug repurposing for inflammatory disorders.
中文翻译:

缺氧诱导因子不仅调节而且是髓细胞治疗的靶点
缺氧描述了细胞水平上有限的氧气供应。骨髓细胞在身体的各个部位都处于缺氧状态,甚至通过在呼吸爆发期间消耗大量氧气而导致缺氧。缺氧诱导因子 (HIF) 是普遍表达的异二聚体转录因子,由氧依赖性 α 和组成型 β 亚基组成。HIF-1α 和 HIF-2α 的稳定性受氧敏感脯氨酰羟化酶 (PHD) 的调节。HIF-1α 和 HIF-2α 会改变先天免疫反应并且是上下文相关的。我们提供了 HIF 发现的历史视角,讨论了 HIF 通路的分子成分,以及 HIF 依赖性机制如何修改骨髓细胞功能。HIF 通过上调无氧糖酵解使骨髓细胞适应缺氧。除了影响新陈代谢,HIF 控制趋化性、吞噬作用、脱颗粒、氧化爆发和细胞凋亡。HIF-1α 使骨髓细胞能够有效防御感染。HIF-2α 通过促进肿瘤相关的骨髓细胞冬眠来延迟炎症消退并降低抗肿瘤作用。PHDs 不仅控制 HIF 降解,还调节先天免疫细胞和适应性免疫细胞之间的串扰,从而抑制自身免疫。HIF 修饰药物化合物正在进入临床实践。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。HIF-2α 通过促进肿瘤相关的骨髓细胞冬眠来延迟炎症消退并降低抗肿瘤作用。PHDs 不仅控制 HIF 降解,还调节先天免疫细胞和适应性免疫细胞之间的串扰,从而抑制自身免疫。HIF 修饰药物化合物正在进入临床实践。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。HIF-2α 通过促进肿瘤相关的骨髓细胞冬眠来延迟炎症消退并降低抗肿瘤作用。PHD 不仅控制 HIF 降解,还调节先天性和适应性免疫细胞之间的串扰,从而抑制自身免疫。HIF 修饰药物化合物正在进入临床实践。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。
更新日期:2020-10-18
中文翻译:

缺氧诱导因子不仅调节而且是髓细胞治疗的靶点
缺氧描述了细胞水平上有限的氧气供应。骨髓细胞在身体的各个部位都处于缺氧状态,甚至通过在呼吸爆发期间消耗大量氧气而导致缺氧。缺氧诱导因子 (HIF) 是普遍表达的异二聚体转录因子,由氧依赖性 α 和组成型 β 亚基组成。HIF-1α 和 HIF-2α 的稳定性受氧敏感脯氨酰羟化酶 (PHD) 的调节。HIF-1α 和 HIF-2α 会改变先天免疫反应并且是上下文相关的。我们提供了 HIF 发现的历史视角,讨论了 HIF 通路的分子成分,以及 HIF 依赖性机制如何修改骨髓细胞功能。HIF 通过上调无氧糖酵解使骨髓细胞适应缺氧。除了影响新陈代谢,HIF 控制趋化性、吞噬作用、脱颗粒、氧化爆发和细胞凋亡。HIF-1α 使骨髓细胞能够有效防御感染。HIF-2α 通过促进肿瘤相关的骨髓细胞冬眠来延迟炎症消退并降低抗肿瘤作用。PHDs 不仅控制 HIF 降解,还调节先天免疫细胞和适应性免疫细胞之间的串扰,从而抑制自身免疫。HIF 修饰药物化合物正在进入临床实践。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。HIF-2α 通过促进肿瘤相关的骨髓细胞冬眠来延迟炎症消退并降低抗肿瘤作用。PHDs 不仅控制 HIF 降解,还调节先天免疫细胞和适应性免疫细胞之间的串扰,从而抑制自身免疫。HIF 修饰药物化合物正在进入临床实践。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。HIF-2α 通过促进肿瘤相关的骨髓细胞冬眠来延迟炎症消退并降低抗肿瘤作用。PHD 不仅控制 HIF 降解,还调节先天性和适应性免疫细胞之间的串扰,从而抑制自身免疫。HIF 修饰药物化合物正在进入临床实践。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。目前的适应症包括肾性贫血和某些癌症。应考虑对骨髓细胞的有益和不利影响,并可能导致炎症性疾病的药物重新利用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号