当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Transition Metals on the Oxygen Reduction Reaction Activity at Metal‐N3/C Active Sites
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-10-18 , DOI: 10.1002/celc.202000954 Holly M. Fruehwald 1 , Iraklii I. Ebralidze 1 , Olena V. Zenkina 1 , E. Bradley Easton 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-10-18 , DOI: 10.1002/celc.202000954 Holly M. Fruehwald 1 , Iraklii I. Ebralidze 1 , Olena V. Zenkina 1 , E. Bradley Easton 1
Affiliation
|
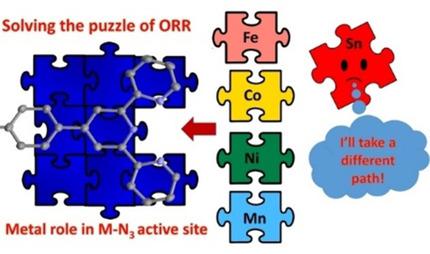
The roles of various transition and post‐transition metals in model non‐precious‐metal catalysts for the oxygen reduction reaction (ORR) are reported after being prepared via a molecularly defined terpyridine unit covalently attached to a carbon black support. We previously reported the use of a terpyridine‐modified iron‐based catalyst that allowed for the controlled deposition of highly active nitrogen functionalities on the carbon support, which adopts a Fe−N3/C active site formation. In this work, we expand on this idea by altering the metal center in the predefined active sites M−N3/C and compare the ORR reactivity of the isostructural set of catalysts, where M=Fe, Co, Ni, Mn, and Sn. The results show that the iron‐based material was the most active catalyst in acid, whereas the cobalt‐based catalyst was most active in base. In addition, nickel‐ and manganese‐based materials showed promising activity for the ORR in both acidic and basic media. We demonstrate that, with a suitable templating bis‐chelating nitrogenous ligand, the M−N3/C active site geometry is adopted with a wide range of non‐precious‐metal centers on a Vulcan carbon surface, and the resulting catalysts are ORR active in a range of conditions, further confirming the tunability and versatility of the N3 site. As expected, post‐transition metals, such as tin, do not coordinate to the N3 nitrogenous ligand under synthetic conditions and deposits tin oxides(s). This study confirms the generality of the phenomenon of M−N3/C as an ORR catalytic site.
中文翻译:

过渡金属对N3 / C活性位处氧还原反应活性的影响
通过共价连接到炭黑载体上的分子定义的三联吡啶单元制备后,报道了各种过渡金属和过渡金属在氧还原反应(ORR)的模型非贵金属催化剂中的作用。我们之前曾报道过使用三联吡啶修饰的铁基催化剂,该催化剂可将高活性氮官能团控制沉积在碳载体上,从而采用Fe-N 3 / C活性位点形成。在这项工作中,我们通过更改预定义的活动站点M-N 3中的金属中心来扩展此思想/ C并比较同构催化剂组的ORR反应性,其中M = Fe,Co,Ni,Mn和Sn。结果表明,铁基材料是酸中活性最高的催化剂,而钴基催化剂在碱中活性最高。此外,镍和锰基材料在酸性和碱性介质中对ORR均显示出良好的活性。我们证明,采用合适的模板化双螯合含氮配体,采用M-N 3 / C活性位点几何构型,在Vulcan碳表面上具有广泛的非贵金属中心,并且所得催化剂具有ORR活性在一系列条件下,进一步证实了N 3位点的可调性和多功能性。不出所料,过渡金属(例如锡)不与N 3配位合成条件下的含氮配体,并沉积氧化锡。这项研究证实了作为ORR催化位点的M-N 3 / C现象的普遍性。
更新日期:2020-10-18
中文翻译:

过渡金属对N3 / C活性位处氧还原反应活性的影响
通过共价连接到炭黑载体上的分子定义的三联吡啶单元制备后,报道了各种过渡金属和过渡金属在氧还原反应(ORR)的模型非贵金属催化剂中的作用。我们之前曾报道过使用三联吡啶修饰的铁基催化剂,该催化剂可将高活性氮官能团控制沉积在碳载体上,从而采用Fe-N 3 / C活性位点形成。在这项工作中,我们通过更改预定义的活动站点M-N 3中的金属中心来扩展此思想/ C并比较同构催化剂组的ORR反应性,其中M = Fe,Co,Ni,Mn和Sn。结果表明,铁基材料是酸中活性最高的催化剂,而钴基催化剂在碱中活性最高。此外,镍和锰基材料在酸性和碱性介质中对ORR均显示出良好的活性。我们证明,采用合适的模板化双螯合含氮配体,采用M-N 3 / C活性位点几何构型,在Vulcan碳表面上具有广泛的非贵金属中心,并且所得催化剂具有ORR活性在一系列条件下,进一步证实了N 3位点的可调性和多功能性。不出所料,过渡金属(例如锡)不与N 3配位合成条件下的含氮配体,并沉积氧化锡。这项研究证实了作为ORR催化位点的M-N 3 / C现象的普遍性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号