Molecular Metabolism ( IF 7.0 ) Pub Date : 2020-10-19 , DOI: 10.1016/j.molmet.2020.101103 Anna Sofie Husted 1 , Jeppe H Ekberg 1 , Emma Tripp 2 , Tinne A D Nissen 1 , Stijn Meijnikman 3 , Shannon L O'Brien 2 , Trond Ulven 4 , Yair Acherman 5 , Sjoerd C Bruin 5 , Max Nieuwdorp 3 , Zach Gerhart-Hines 1 , Davide Calebiro 2 , Lars O Dragsted 6 , Thue W Schwartz 1
|
Objectives
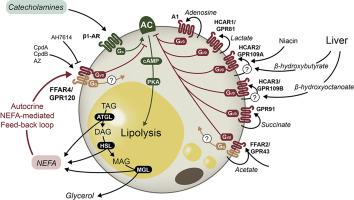
Long-chain fatty acids (LCFAs) released from adipocytes inhibit lipolysis through an unclear mechanism. We hypothesized that the LCFA receptor, FFAR4 (GPR120), which is highly expressed in adipocytes, may be involved in this feedback regulation.
Methods and results
Liquid chromatography mass spectrometry (LC-MS) analysis of conditioned media from isoproterenol-stimulated primary cultures of murine and human adipocytes demonstrated that most of the released non-esterified free fatty acids (NEFAs) are known agonists for FFAR4. In agreement with this, conditioned medium from isoproterenol-treated adipocytes stimulated signaling strongly in FFAR4 transfected COS-7 cells as opposed to non-transfected control cells. In transfected 3T3-L1 cells, FFAR4 agonism stimulated Gi- and Go-mini G protein binding more strongly than Gq, effects which were blocked by the selective FFAR4 antagonist AH7614. In primary cultures of murine white adipocytes, the synthetic, selective FFAR4 agonist CpdA inhibited isoproterenol-induced intracellular cAMP accumulation in a manner similar to the antilipolytic control agent nicotinic acid acting through another receptor, HCAR2. In vivo, oral gavage with the synthetic, specific FFAR4 agonist CpdB decreased the level of circulating NEFAs in fasting lean mice to a similar degree as nicotinic acid. In agreement with the identified anti-lipolytic effect of FFAR4, plasma NEFAs and glycerol were increased in FFAR4-deficient mice as compared to littermate controls despite having elevated insulin levels, and cAMP accumulation in primary adipocyte cultures was augmented by treatment with the FFAR4 antagonist conceivably by blocking the stimulatory tone of endogenous NEFAs on FFAR4.
Conclusions
In white adipocytes, FFAR4 functions as an NEFA-activated, autocrine, negative feedback regulator of lipolysis by decreasing cAMP though Gi-mediated signaling.
中文翻译:

WAT 中 FFAR4/GPR120 通过感测 NEFA 对脂肪分解进行自分泌负反馈调节
目标
脂肪细胞释放的长链脂肪酸(LCFA)通过一种尚不清楚的机制抑制脂肪分解。我们假设在脂肪细胞中高表达的 LCFA 受体 FFAR4 (GPR120) 可能参与这种反馈调节。
方法和结果
对异丙肾上腺素刺激的鼠和人脂肪细胞原代培养物的条件培养基进行液相色谱质谱 (LC-MS) 分析表明,大多数释放的非酯化游离脂肪酸 (NEFA) 是已知的 FFAR4 激动剂。与此一致的是,与未转染的对照细胞相比,异丙肾上腺素处理的脂肪细胞的条件培养基强烈刺激FFAR4转染的COS-7细胞中的信号传导。在转染的 3T3-L1 细胞中,FFAR4 激动作用比 Gq 更强烈地刺激 Gi- 和 Go-mini G 蛋白结合,但该作用被选择性 FFAR4 拮抗剂 AH7614 阻断。在小鼠白色脂肪细胞的原代培养物中,合成的选择性 FFAR4 激动剂 CpdA 抑制异丙肾上腺素诱导的细胞内 cAMP 积累,其方式类似于抗脂解控制剂烟酸通过另一种受体 HCAR2 发挥作用。在体内,口服合成的特异性 FFAR4 激动剂 CpdB 可以降低禁食瘦小鼠的循环 NEFA 水平,其程度与烟酸相似。与已确定的 FFAR4 抗脂肪分解作用一致,尽管胰岛素水平升高,但 FFAR4 缺陷小鼠的血浆 NEFA 和甘油与同窝对照小鼠相比有所增加,并且可以想象,通过 FFAR4 拮抗剂治疗,原代脂肪细胞培养物中的 cAMP 积累增加通过阻断内源性 NEFA 对 FFAR4 的刺激音。
结论
在白色脂肪细胞中,FFAR4 通过 Gi 介导的信号传导降低 cAMP,充当 NEFA 激活的自分泌负反馈脂肪分解调节剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号