European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-10-17 , DOI: 10.1016/j.ejmech.2020.112951 Gui-Bin Liang 1 , Jian-Hua Wei 2 , Hong Jiang 2 , Ri-Zhen Huang 1 , Jing-Ting Qin 2 , Hui-Ling Wang 2 , Heng-Shan Wang 3 , Ye Zhang 4
|
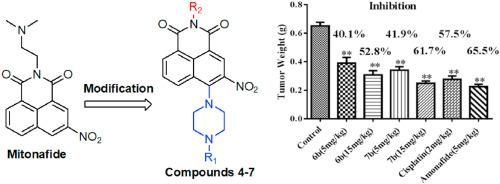
Four series of new 3-nitro naphthalimides derivatives, 4(4a‒4f), 5(5a‒5i), 6(6a‒6e) and 7 (7a‒7j), were designed and synthesized as antitumor agents. Methyl thiazolyl tetrazolium (MTT) screening assay results revealed that some compounds displayed effective in vitro antiproliferative activity on SMMC-7721, T24, SKOV-3, A549 and MGC-803 cancer cell lines in comparison with 5-fluorouracil (5-FU), mitonafide and amonafide. Nude mouse xenotransplantation model assay results indicated that compounds 6b and 7b exhibited good in vivo antiproliferative activity in MGC-803 xenografts in comparison with amonafide and cisplatin, suggesting that compounds 6b and 7b could be good candidates for antitumor agents. Gel electrophoresis assay indicated that DNA and Topo I were the potential targets of compounds 6b and 7b, and comet assay confirmed that compounds 6b and 7b could induce DNA damage, while the further study showed that the 6b- and 7b-induced DNA damage was accompanied by the upregulation of p-ATM, P-Chk2, Cdc25A and p-H2AX. Cell cycle arrest studies demonstrated that compounds 6b and 7b arrested the cell cycle at the S phase, accompanied by the upregulation of the expression levels of the antioncogene p21 and the down-regulation of the expression levels of cyclin E. Apoptosis assays indicated that compounds 6b and 7b caused the apoptosis of tumor cells along with the upregulation of the expression of Bax, caspase-3, caspase-9 and PARP and the downregulation of Bcl-2. These mechanistic studies suggested that compounds 6b and 7b exerted their antitumor activity by targeting to DNA, thereby inducing DNA damage and Topo I inhibition, and consequently causing S stage arrest and the induction of apoptosis.
中文翻译:

靶向核DNA的新型1,8-萘酰亚胺衍生物的设计、合成和抗肿瘤评价
设计并合成了四个系列的新型3-硝基萘酰亚胺衍生物4 ( 4a-4f )、 5 ( 5a-5i )、 6 ( 6a-6e )和7 ( 7a-7j )作为抗肿瘤药物。甲基噻唑基四唑 (MTT) 筛选试验结果显示,与 5-氟尿嘧啶 (5-FU) 相比,一些化合物对 SMMC-7721、T24、SKOV-3、A549 和 MGC-803 癌细胞系表现出有效的体外抗增殖活性。米托那菲和阿莫那菲。裸鼠异种移植模型测定结果表明,与amonafide和顺铂相比,化合物6b和7b在MGC-803异种移植物中表现出良好的体内抗增殖活性,表明化合物6b和7b可能是抗肿瘤药物的良好候选者。凝胶电泳分析表明DNA和Topo I是化合物6b和7b的潜在靶标,彗星实验证实化合物6b和7b可以诱导DNA损伤,而进一步的研究表明6b和7b诱导的DNA损伤是伴随的。通过 p-ATM、P-Chk2、Cdc25A 和 p-H2AX 的上调。细胞周期阻滞研究表明,化合物6b和7b将细胞周期阻滞在S期,同时上调抑癌基因p21的表达水平和下调细胞周期蛋白E的表达水平。 细胞凋亡测定表明,化合物6b和7b引起肿瘤细胞凋亡,同时上调Bax、caspase-3、caspase-9和PARP的表达以及下调Bcl-2的表达。这些机制研究表明,化合物6b和7b通过靶向DNA发挥抗肿瘤活性,从而诱导DNA损伤和Topo I抑制,从而引起S期停滞并诱导细胞凋亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号