当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies Inform Design of Improved Ti(salen) Catalysts for Enantioselective [3 + 2] Cycloaddition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-16 , DOI: 10.1021/jacs.0c07128 Sophia G. Robinson 1 , Xiangyu Wu 2 , Binyang Jiang 2 , Matthew S. Sigman 1 , Song Lin 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-10-16 , DOI: 10.1021/jacs.0c07128 Sophia G. Robinson 1 , Xiangyu Wu 2 , Binyang Jiang 2 , Matthew S. Sigman 1 , Song Lin 2
Affiliation
|
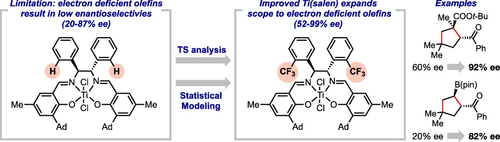
Ti(salen) complexes catalyze the asymmetric [3 + 2] cycloaddition of cyclopropyl ketones with alkenes. While high enantioselectivities are achieved with electron-rich alkenes, electron-deficient alkenes are less selective. Herein, we describe mechanistic studies to understand the origins of catalyst and substrate trends in an effort to identify a more general catalyst. Density functional theory (DFT) calculations of the selectivity determining transition state revealed the origin of stereochemical control to be catalyst distortion, which is largely influenced by the chiral backbone and adamantyl groups on the salicylaldehyde moieties. While substitution of the adamantyl groups was detrimental to the enantioselectivity, mechanistic information guided the development of a set of eight new Ti(salen) catalysts with modified diamine backbones. These catalysts were evaluated with four electron-deficient alkenes to develop a three-parameter statistical model relating enantioselectivity to physical organic parameters. This statistical model is capable of quantitative prediction of enantioselectivity with structurally diverse alkenes. These mechanistic insights assisted the discovery of a new Ti(salen) catalyst, which substantially expanded the reaction scope and significantly improved the enantioselectivity of synthetically interesting building blocks.
中文翻译:

用于对映选择性 [3 + 2] 环加成的改进 Ti(salen) 催化剂的机理研究
Ti(salen) 配合物催化环丙基酮与烯烃的不对称 [3 + 2] 环加成。虽然富电子烯烃具有高对映选择性,但缺电子烯烃的选择性较低。在此,我们描述了机理研究以了解催化剂的起源和底物趋势,以努力确定更通用的催化剂。决定过渡态的选择性的密度泛函理论 (DFT) 计算揭示了立体化学控制的起源是催化剂变形,这在很大程度上受水杨醛部分上的手性骨架和金刚烷基的影响。虽然金刚烷基的取代不利于对映选择性,但机械信息指导了一组八种具有改性二胺主链的新型 Ti(salen) 催化剂的开发。用四种缺电子烯烃对这些催化剂进行了评估,以开发出一种将对映选择性与物理有机参数相关联的三参数统计模型。该统计模型能够定量预测具有不同结构的烯烃的对映选择性。这些机理见解有助于发现一种新的 Ti(salen) 催化剂,该催化剂大大扩展了反应范围并显着提高了合成有趣结构单元的对映选择性。
更新日期:2020-10-16
中文翻译:

用于对映选择性 [3 + 2] 环加成的改进 Ti(salen) 催化剂的机理研究
Ti(salen) 配合物催化环丙基酮与烯烃的不对称 [3 + 2] 环加成。虽然富电子烯烃具有高对映选择性,但缺电子烯烃的选择性较低。在此,我们描述了机理研究以了解催化剂的起源和底物趋势,以努力确定更通用的催化剂。决定过渡态的选择性的密度泛函理论 (DFT) 计算揭示了立体化学控制的起源是催化剂变形,这在很大程度上受水杨醛部分上的手性骨架和金刚烷基的影响。虽然金刚烷基的取代不利于对映选择性,但机械信息指导了一组八种具有改性二胺主链的新型 Ti(salen) 催化剂的开发。用四种缺电子烯烃对这些催化剂进行了评估,以开发出一种将对映选择性与物理有机参数相关联的三参数统计模型。该统计模型能够定量预测具有不同结构的烯烃的对映选择性。这些机理见解有助于发现一种新的 Ti(salen) 催化剂,该催化剂大大扩展了反应范围并显着提高了合成有趣结构单元的对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号