当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of 2,3-Epoxypropyl Neodecanoate: Process Optimization and Mechanism Discussion
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-10-16 , DOI: 10.1021/acs.iecr.0c02906 Zifei Yan 1 , Jian Deng 1 , Yuchao Chen 1 , Guangsheng Luo 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-10-16 , DOI: 10.1021/acs.iecr.0c02906 Zifei Yan 1 , Jian Deng 1 , Yuchao Chen 1 , Guangsheng Luo 1
Affiliation
|
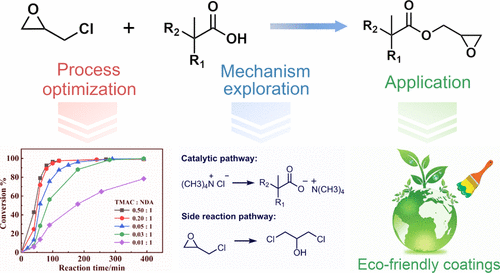
2,3-Epoxypropyl neodecanoate (EPDA) is an important synthon of eco-friendly coatings, and its preparation methods and the relevant reaction mechanisms remain to be further studied. In this work, the two-step preparation method of EPDA is investigated. Under the optimized process conditions in the acidolysis reaction, 99% conversion of neodecanoic acid and 91–93% effective utilization rate of epichlorohydrin could be realized. In the following ring-closure reaction, the two-step alkali treatment was applied, and it can significantly reduce the treatment time and improve the yield of EPDA by 3–5%. Besides, the mechanism of the acidolysis reaction catalyzed by tetramethylammonium chloride is proposed. The two chemical reaction pathways are put forward to explain the formation of side product dichloropropanol. Two effective ways to control the side reactions are suggested, including the control of temperature in the range of 70–90 °C and reducing the concentration of inorganic chlorine in the reaction system.
中文翻译:

新癸酸2,3-环氧丙酯的制备:工艺优化及机理探讨
2,3-环氧丙基新癸酸酯(EPDA)是一种环保型涂料的重要合成物,其制备方法和相关的反应机理还有待进一步研究。在这项工作中,研究了EPDA的两步制备方法。在酸解反应的最佳工艺条件下,新癸酸的转化率为99%,表氯醇的有效利用率为91-93%。在随后的闭环反应中,进行了两步碱处理,它可以显着减少处理时间并使EPDA的收率提高3-5%。此外,提出了四甲基氯化铵催化酸解反应的机理。提出了两种化学反应途径来解释副产物二氯丙醇的形成。
更新日期:2020-10-29
中文翻译:

新癸酸2,3-环氧丙酯的制备:工艺优化及机理探讨
2,3-环氧丙基新癸酸酯(EPDA)是一种环保型涂料的重要合成物,其制备方法和相关的反应机理还有待进一步研究。在这项工作中,研究了EPDA的两步制备方法。在酸解反应的最佳工艺条件下,新癸酸的转化率为99%,表氯醇的有效利用率为91-93%。在随后的闭环反应中,进行了两步碱处理,它可以显着减少处理时间并使EPDA的收率提高3-5%。此外,提出了四甲基氯化铵催化酸解反应的机理。提出了两种化学反应途径来解释副产物二氯丙醇的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号