当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self‐assembly of hairpin peptides mediated by Cu(II) ion: Effect of amino acid sequence
Peptide Science ( IF 1.5 ) Pub Date : 2020-10-17 , DOI: 10.1002/pep2.24208 Jiqian Wang 1 , Chengdong Wang 2 , Yanqing Ge 1 , Yawei Sun 1 , Dong Wang 1 , Hai Xu 1
Peptide Science ( IF 1.5 ) Pub Date : 2020-10-17 , DOI: 10.1002/pep2.24208 Jiqian Wang 1 , Chengdong Wang 2 , Yanqing Ge 1 , Yawei Sun 1 , Dong Wang 1 , Hai Xu 1
Affiliation
|
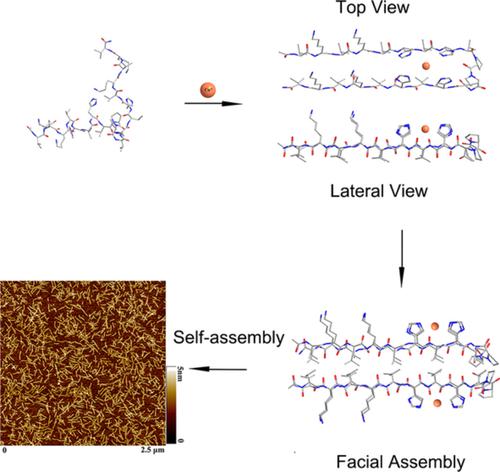
The alteration of amino acid register in peptides could affect their folding properties and thus functions. The effect of sequence in metal ion induced folding of peptide/protein is important in the design of new functional protein structures, such as biomimetics. In this paper, a set of hairpin peptides with identical composition but different histidine locations have been designed to exploit the metal ion‐mediated peptide intramolecular folding transformation through the adjustment of histidine and lysine residue locations. The results demonstrated that the histidine positions in peptide sequence could dramatically affect the coordination modes between peptides and Cu(II) ion. The Cu(II) coordination could affect the secondary structure transformation and self‐assembly along with electrostatic interaction and hydrogen bonding synergistically. Three molecules with VHVH sequence at each side of the turn segment (VDPPT) were apt to coordinate with Cu(II) ion with three or four histidines. The closer the VHVH motifs were to the turn segment, the more easier the molecules were to form β sheet structure with the promotion of Cu(II) ions. These results demonstrated that the purposeful location of key amino acids in peptides could achieve the controllable conformation transformation of hairpin peptides, which provides useful strategies for biomimetic design, such as metalloenzymes.
中文翻译:

Cu(II)离子介导的发夹肽的自组装:氨基酸序列的影响
肽中氨基酸寄存器的改变可能会影响其折叠特性,从而影响其功能。序列在金属离子诱导的肽/蛋白质折叠中的作用对于设计新的功能性蛋白质结构(例如仿生体)非常重要。本文设计了一组组成相同但组氨酸位置不同的发夹肽,以通过调节组氨酸和赖氨酸残基的位置来利用金属离子介导的肽的分子内折叠转化。结果表明,肽序列中的组氨酸位置可显着影响肽与Cu(II)离子之间的配位方式。Cu(II)配位可能会影响二级结构的转变和自组装以及静电相互作用和氢键的协同作用。D PPT)易于与带有三个或四个组氨酸的Cu(II)离子配位。VHVH基序越靠近转弯区段,分子越容易形成具有Cu(II)离子促进作用的β片层结构。这些结果表明,关键氨基酸在肽中的有目的地定位可以实现发夹肽的可控构象转化,这为仿生设计提供了有用的策略,例如金属酶。
更新日期:2020-10-17
中文翻译:

Cu(II)离子介导的发夹肽的自组装:氨基酸序列的影响
肽中氨基酸寄存器的改变可能会影响其折叠特性,从而影响其功能。序列在金属离子诱导的肽/蛋白质折叠中的作用对于设计新的功能性蛋白质结构(例如仿生体)非常重要。本文设计了一组组成相同但组氨酸位置不同的发夹肽,以通过调节组氨酸和赖氨酸残基的位置来利用金属离子介导的肽的分子内折叠转化。结果表明,肽序列中的组氨酸位置可显着影响肽与Cu(II)离子之间的配位方式。Cu(II)配位可能会影响二级结构的转变和自组装以及静电相互作用和氢键的协同作用。D PPT)易于与带有三个或四个组氨酸的Cu(II)离子配位。VHVH基序越靠近转弯区段,分子越容易形成具有Cu(II)离子促进作用的β片层结构。这些结果表明,关键氨基酸在肽中的有目的地定位可以实现发夹肽的可控构象转化,这为仿生设计提供了有用的策略,例如金属酶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号