当前位置:
X-MOL 学术
›
Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homologous Recombination Offers Advantages over Transposition‐Based Systems to Generate Recombinant Baculovirus for Adeno‐Associated Viral Vector Production
Biotechnology Journal ( IF 3.2 ) Pub Date : 2020-10-16 , DOI: 10.1002/biot.202000014 Aurélien Jacob 1 , Laurie Brun 1 , Paloma Jiménez Gil 1 , Lucie Ménard 1 , Mohammed Bouzelha 1 , Frédéric Broucque 1 , Aline Roblin 1 , Luk H. Vandenberghe 2, 3, 4, 5 , Oumeya Adjali 1 , Cécile Robin 1 , Achille François 1 , Véronique Blouin 1 , Magalie Penaud‐Budloo 1 , Eduard Ayuso 1
Biotechnology Journal ( IF 3.2 ) Pub Date : 2020-10-16 , DOI: 10.1002/biot.202000014 Aurélien Jacob 1 , Laurie Brun 1 , Paloma Jiménez Gil 1 , Lucie Ménard 1 , Mohammed Bouzelha 1 , Frédéric Broucque 1 , Aline Roblin 1 , Luk H. Vandenberghe 2, 3, 4, 5 , Oumeya Adjali 1 , Cécile Robin 1 , Achille François 1 , Véronique Blouin 1 , Magalie Penaud‐Budloo 1 , Eduard Ayuso 1
Affiliation
|
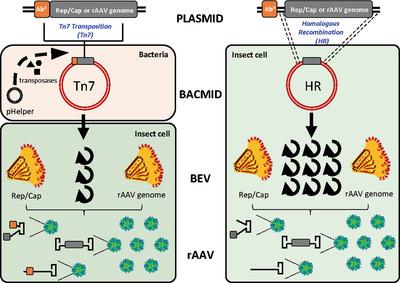
Viral vectors have a great potential for gene delivery, but manufacturing is a big challenge for the industry. The baculovirus‐insect cell is one of the most scalable platforms to produce recombinant adeno‐associated virus (rAAV) vectors. The standard procedure to generate recombinant baculovirus is based on Tn7 transposition which is time‐consuming and suffers technical constraints. Moreover, baculoviral sequences adjacent to the AAV ITRs are preferentially encapsidated into the rAAV vector particles. This observation raises concerns about safety due to the presence of bacterial and antibiotic resistance coding sequences with a Tn7‐mediated system for the construction of baculoviruses reagents. Here, a faster and safer method based on homologous recombination (HR) is investigated. First, the functionality of the inserted cassette and the absence of undesirable genes into HR‐derived baculoviral genomes are confirmed. Strikingly, it is found that the exogenous cassette showed increased stability over passages when using the HR system. Finally, both materials generated high rAAV vector genome titers, with the advantage of the HR system being exempted from undesirable bacterial genes which provides an additional level of safety for its manufacturing. Overall, this study highlights the importance of the upstream process and starting biologic materials to generate safer rAAV biotherapeutic products.
中文翻译:

同源重组提供了优于基于换位的系统的优势,可产生重组杆状病毒用于腺相关病毒载体的生产
病毒载体在基因递送方面具有巨大潜力,但是制造对工业来说是巨大的挑战。杆状病毒昆虫细胞是生产重组腺相关病毒(rAAV)载体的最具扩展性的平台之一。产生重组杆状病毒的标准程序是基于Tn7转座的,这既费时又受技术限制。而且,与AAV ITR相邻的杆状病毒序列优选被衣壳化到rAAV载体颗粒中。由于Tn7介导的杆状病毒试剂构建系统中存在细菌和抗生素抗性编码序列,因此该观察结果引起了对安全性的关注。在这里,研究了一种基于同源重组(HR)的更快,更安全的方法。第一,可以确定插入的盒的功能以及HR衍生的杆状病毒基因组中不存在不需要的基因。令人惊讶地,发现当使用HR系统时,外源盒在通道中显示出增加的稳定性。最后,两种材料均产生高rAAV载体基因组滴度,而HR系统的优势在于可免除有害细菌基因,从而为其生产提供了更高的安全性。总的来说,这项研究突出了上游过程和起始生物材料以产生更安全的rAAV生物治疗产品的重要性。两种材料均产生高rAAV载体基因组滴度,并且HR系统免于不需要的细菌基因的优势,这为其制造提供了更高的安全性。总的来说,这项研究突出了上游过程和起始生物材料以产生更安全的rAAV生物治疗产品的重要性。两种材料均产生高rAAV载体基因组滴度,并且HR系统免于不需要的细菌基因的优势,这为其制造提供了更高的安全性。总的来说,这项研究突出了上游过程和起始生物材料以产生更安全的rAAV生物治疗产品的重要性。
更新日期:2020-10-16
中文翻译:

同源重组提供了优于基于换位的系统的优势,可产生重组杆状病毒用于腺相关病毒载体的生产
病毒载体在基因递送方面具有巨大潜力,但是制造对工业来说是巨大的挑战。杆状病毒昆虫细胞是生产重组腺相关病毒(rAAV)载体的最具扩展性的平台之一。产生重组杆状病毒的标准程序是基于Tn7转座的,这既费时又受技术限制。而且,与AAV ITR相邻的杆状病毒序列优选被衣壳化到rAAV载体颗粒中。由于Tn7介导的杆状病毒试剂构建系统中存在细菌和抗生素抗性编码序列,因此该观察结果引起了对安全性的关注。在这里,研究了一种基于同源重组(HR)的更快,更安全的方法。第一,可以确定插入的盒的功能以及HR衍生的杆状病毒基因组中不存在不需要的基因。令人惊讶地,发现当使用HR系统时,外源盒在通道中显示出增加的稳定性。最后,两种材料均产生高rAAV载体基因组滴度,而HR系统的优势在于可免除有害细菌基因,从而为其生产提供了更高的安全性。总的来说,这项研究突出了上游过程和起始生物材料以产生更安全的rAAV生物治疗产品的重要性。两种材料均产生高rAAV载体基因组滴度,并且HR系统免于不需要的细菌基因的优势,这为其制造提供了更高的安全性。总的来说,这项研究突出了上游过程和起始生物材料以产生更安全的rAAV生物治疗产品的重要性。两种材料均产生高rAAV载体基因组滴度,并且HR系统免于不需要的细菌基因的优势,这为其制造提供了更高的安全性。总的来说,这项研究突出了上游过程和起始生物材料以产生更安全的rAAV生物治疗产品的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号