Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomic structure of, and valine binding to the regulatory ACT domain of the Mycobacterium tuberculosis Rel protein
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-17 , DOI: 10.1111/febs.15600 Joon Shin 1 , Bharti Singal 1 , Malathy Sony Subramanian Manimekalai 1 , Ming Wei Chen 1 , Priya Ragunathan 1 , Gerhard Grüber 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-17 , DOI: 10.1111/febs.15600 Joon Shin 1 , Bharti Singal 1 , Malathy Sony Subramanian Manimekalai 1 , Ming Wei Chen 1 , Priya Ragunathan 1 , Gerhard Grüber 1
Affiliation
|
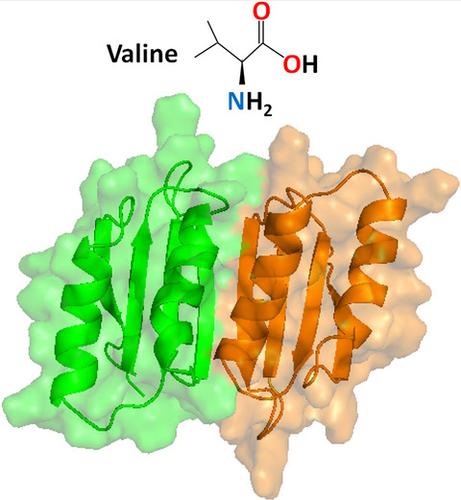
The stringent response, regulated by the bifunctional (p)ppGpp synthetase/hydrolase Rel in mycobacteria, is critical for long‐term survival of the drug‐tolerant dormant state of Mycobacterium tuberculosis. During amino acid starvation, MtRel senses a drop in amino acid concentration and synthesizes the messengers pppGpp and ppGpp, collectively called (p)ppGpp. Here, we investigate the role of the regulatory ‘Aspartokinase, Chorismate mutase and TyrA’ (ACT) domain in MtRel. Using NMR spectroscopy approaches, we report the high‐resolution structure of dimeric MtRel ACT which selectively binds to valine out of all other branched‐chain amino acids tested. A set of MtRel ACT mutants were generated to identify the residues required for maintaining the head‐to‐tail dimer. Through NMR titrations, we determined the crucial residues for binding of valine and show structural rearrangement of the MtRel ACT dimer in the presence of valine. This study suggests the direct involvement of amino acids in (p)ppGpp accumulation mediated by MtRel independent to interactions with stalled ribosomes.
中文翻译:

结核分枝杆菌Rel蛋白调节性ACT结构域的原子结构和缬氨酸结合
严格的响应,由双官能(p)的调节ppGpp的合成酶/水解酶的Rel在分枝杆菌,是用于药物耐受休眠状态的长期生存的关键结核分枝杆菌。在氨基酸饥饿期间,Mt Rel感知到氨基酸浓度的下降并合成了信使pppGpp和ppGpp(统称为(p)ppGpp)。在这里,我们调查Mt Rel中的调节性'天冬氨酸激酶,Chorismate突变酶和TyrA'(ACT)域的作用。使用NMR光谱方法,我们报告了二聚体Mt Rel ACT的高分辨率结构,该结构可选择性结合所有测试的其他支链氨基酸中的缬氨酸。一套山产生Rel ACT突变体以鉴定维持头尾二聚体所需的残基。通过NMR滴定,我们确定了与缬氨酸结合的关键残基,并在存在缬氨酸的情况下显示了Mt Rel ACT二聚体的结构重排。这项研究表明氨基酸直接参与由Mt Rel介导的(p)ppGpp积累,而与与失速核糖体的相互作用无关。
更新日期:2020-10-17
中文翻译:

结核分枝杆菌Rel蛋白调节性ACT结构域的原子结构和缬氨酸结合
严格的响应,由双官能(p)的调节ppGpp的合成酶/水解酶的Rel在分枝杆菌,是用于药物耐受休眠状态的长期生存的关键结核分枝杆菌。在氨基酸饥饿期间,Mt Rel感知到氨基酸浓度的下降并合成了信使pppGpp和ppGpp(统称为(p)ppGpp)。在这里,我们调查Mt Rel中的调节性'天冬氨酸激酶,Chorismate突变酶和TyrA'(ACT)域的作用。使用NMR光谱方法,我们报告了二聚体Mt Rel ACT的高分辨率结构,该结构可选择性结合所有测试的其他支链氨基酸中的缬氨酸。一套山产生Rel ACT突变体以鉴定维持头尾二聚体所需的残基。通过NMR滴定,我们确定了与缬氨酸结合的关键残基,并在存在缬氨酸的情况下显示了Mt Rel ACT二聚体的结构重排。这项研究表明氨基酸直接参与由Mt Rel介导的(p)ppGpp积累,而与与失速核糖体的相互作用无关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号