Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One stem cell program to rule them all?
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-16 , DOI: 10.1111/febs.15598 Mallory Wiggans 1, 2 , Bret J Pearson 1, 2, 3
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-16 , DOI: 10.1111/febs.15598 Mallory Wiggans 1, 2 , Bret J Pearson 1, 2, 3
Affiliation
|
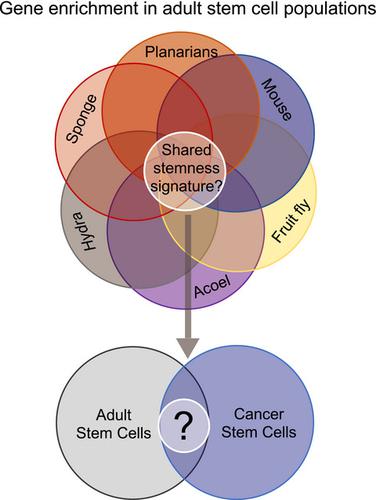
Many species of animals have stem cells that they maintain throughout their lives, which suggests that stem cells are an ancestral feature of all animals. From this, we take the viewpoint that cells with the biological properties of ‘stemness’—self-renewal and multipotency—may share ancestral genetic circuitry. However, in practice is it very difficult to identify and compare stemness gene signatures across diverse animals and large evolutionary distances? First, it is critical to experimentally demonstrate self-renewal and potency. Second, genomic methods must be used to determine specific gene expression in stem cell types compared with non-stem cell types to determine stem cell gene enrichment. Third, gene homology must be mapped between diverse animals across large evolutionary distances. Finally, conserved genes that fulfill these criteria must be tested for role in stem cell function. It is our viewpoint that by comparing stem cell-specific gene signatures across evolution, ancestral programs of stemness can be uncovered, and ultimately, the dysregulation of stemness programs drives the state of cancer stem cells.
中文翻译:

一个干细胞计划来统治所有这些?
许多种类的动物都有干细胞,它们一生都在维持,这表明干细胞是所有动物的祖先特征。由此,我们认为具有“干性”生物学特性——自我更新和多能性——的细胞可能共享祖先的遗传回路。然而,在实践中识别和比较不同动物和大进化距离的干性基因特征是否非常困难?首先,通过实验证明自我更新和效力至关重要。其次,必须使用基因组方法来确定干细胞类型与非干细胞类型相比的特定基因表达,以确定干细胞基因的富集。第三,基因同源性必须在跨越大进化距离的不同动物之间进行映射。最后,必须测试满足这些标准的保守基因在干细胞功能中的作用。我们的观点是,通过比较进化过程中的干细胞特异性基因特征,可以发现干细胞的祖先程序,最终,干细胞程序的失调会驱动癌症干细胞的状态。
更新日期:2020-10-16
中文翻译:

一个干细胞计划来统治所有这些?
许多种类的动物都有干细胞,它们一生都在维持,这表明干细胞是所有动物的祖先特征。由此,我们认为具有“干性”生物学特性——自我更新和多能性——的细胞可能共享祖先的遗传回路。然而,在实践中识别和比较不同动物和大进化距离的干性基因特征是否非常困难?首先,通过实验证明自我更新和效力至关重要。其次,必须使用基因组方法来确定干细胞类型与非干细胞类型相比的特定基因表达,以确定干细胞基因的富集。第三,基因同源性必须在跨越大进化距离的不同动物之间进行映射。最后,必须测试满足这些标准的保守基因在干细胞功能中的作用。我们的观点是,通过比较进化过程中的干细胞特异性基因特征,可以发现干细胞的祖先程序,最终,干细胞程序的失调会驱动癌症干细胞的状态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号