Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modification of the Pseudomonas aeruginosa toxin 2‐heptyl‐1‐hydroxyquinolin‐4(1H)‐one and other secondary metabolites by methyltransferases from mycobacteria
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-16 , DOI: 10.1111/febs.15595 Pascal Sartor 1 , Jonathan Bock 2 , Ulrich Hennecke 2 , Sven Thierbach 1 , Susanne Fetzner 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-10-16 , DOI: 10.1111/febs.15595 Pascal Sartor 1 , Jonathan Bock 2 , Ulrich Hennecke 2 , Sven Thierbach 1 , Susanne Fetzner 1
Affiliation
|
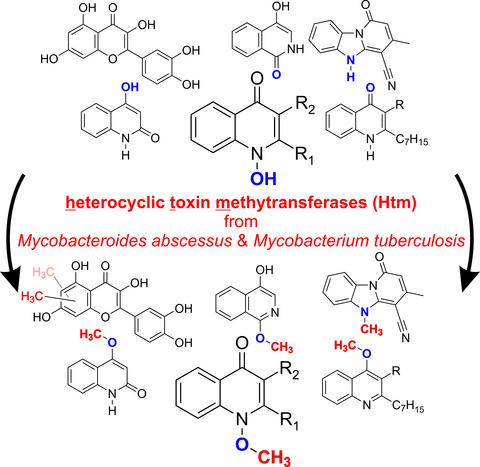
The opportunistic pathogen Pseudomonas aeruginosa, one of the most prevalent species in infections of the cystic fibrosis lung, produces a range of secondary metabolites, among them the respiratory toxin 2‐heptyl‐1‐hydroxyquinolin‐4(1H)‐one (2‐heptyl‐4‐hydroxyquinoline N‐oxide, HQNO). Cultures of the emerging cystic fibrosis pathogen Mycobacteroides abscessus detoxify HQNO by methylating the N‐hydroxy moiety. In this study, the class I methyltransferase MAB_2834c and its orthologue from Mycobacterium tuberculosis, Rv0560c, were identified as HQNO O‐methyltransferases. The P. aeruginosa exoproducts 4‐hydroxyquinolin‐2(1H)‐one (DHQ), 2‐heptylquinolin‐4(1H)‐one (HHQ), and 2‐heptyl‐3‐hydroxyquinolin‐4(1H)‐one (the ‘Pseudomonas quinolone signal’, PQS), some structurally related (iso)quinolones, and the flavonol quercetin were also methylated; however, HQNO was by far the preferred substrate. Both enzymes converted a benzimidazole[1,2‐a]pyridine‐4‐carbonitrile‐based compound, representing the scaffold of antimycobacterial substances, to an N‐methylated derivative. We suggest that these promiscuous methyltransferases, newly termed as heterocyclic toxin methyltransferases (Htm), are involved in cellular response to chemical stress and possibly contribute to resistance of mycobacteria toward antimicrobial natural compounds as well as drugs. Thus, synthetic antimycobacterial agents may be designed to be unamenable to methyl transfer.
中文翻译:

分枝杆菌的甲基转移酶对铜绿假单胞菌毒素2-庚基-1-羟基喹啉-4(1H)-one和其他次级代谢产物的修饰
机会性病原体铜绿假单胞菌是肺囊性纤维化感染中最普遍的物种之一,可产生一系列次级代谢产物,其中包括呼吸道毒素2庚基-1-羟基喹啉-4(1 H)-一(2-庚基-4-羟基喹啉N-氧化物,HQNO)。新兴的囊性纤维化病原体脓肿分支杆菌的培养物通过甲基化N-羟基部分使HQNO解毒。在这项研究中,I类甲基转移酶MAB_2834c及其来自结核分枝杆菌的直向同源物Rv0560c被鉴定为HQNO O-甲基转移酶。在铜绿假单胞菌exoproducts 4-羟基喹啉-2(1ħ) -酮(DHQ),2- heptylquinolin-4(1 ħ) -酮(HHQ)和2-庚基-3-羟基喹啉-4(1 ħ) -酮(以下简称'假单胞菌喹诺酮信号',PQS),一些结构上相关的(异)喹诺酮和黄酮醇槲皮素也被甲基化了;但是,到目前为止,HQNO是首选的底物。两种酶转化的苯并咪唑[1,2一]吡啶-4-甲腈类化合物,占抗分枝杆菌药物的支架,一个Ñ甲基化衍生物。我们建议这些混杂的甲基转移酶,新称为杂环毒素甲基转移酶(Htm),参与细胞对化学应激的反应,并可能导致分枝杆菌对抗菌素天然化合物和药物的耐药性。因此,可以将合成的抗分枝杆菌药设计成不适合甲基转移。
更新日期:2020-10-16
中文翻译:

分枝杆菌的甲基转移酶对铜绿假单胞菌毒素2-庚基-1-羟基喹啉-4(1H)-one和其他次级代谢产物的修饰
机会性病原体铜绿假单胞菌是肺囊性纤维化感染中最普遍的物种之一,可产生一系列次级代谢产物,其中包括呼吸道毒素2庚基-1-羟基喹啉-4(1 H)-一(2-庚基-4-羟基喹啉N-氧化物,HQNO)。新兴的囊性纤维化病原体脓肿分支杆菌的培养物通过甲基化N-羟基部分使HQNO解毒。在这项研究中,I类甲基转移酶MAB_2834c及其来自结核分枝杆菌的直向同源物Rv0560c被鉴定为HQNO O-甲基转移酶。在铜绿假单胞菌exoproducts 4-羟基喹啉-2(1ħ) -酮(DHQ),2- heptylquinolin-4(1 ħ) -酮(HHQ)和2-庚基-3-羟基喹啉-4(1 ħ) -酮(以下简称'假单胞菌喹诺酮信号',PQS),一些结构上相关的(异)喹诺酮和黄酮醇槲皮素也被甲基化了;但是,到目前为止,HQNO是首选的底物。两种酶转化的苯并咪唑[1,2一]吡啶-4-甲腈类化合物,占抗分枝杆菌药物的支架,一个Ñ甲基化衍生物。我们建议这些混杂的甲基转移酶,新称为杂环毒素甲基转移酶(Htm),参与细胞对化学应激的反应,并可能导致分枝杆菌对抗菌素天然化合物和药物的耐药性。因此,可以将合成的抗分枝杆菌药设计成不适合甲基转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号