当前位置:
X-MOL 学术
›
Hum. Brain Mapp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting the sensory feedback within the swallowing network—Reversing artificially induced pharyngolaryngeal hypesthesia by central and peripheral stimulation strategies
Human Brain Mapping ( IF 3.5 ) Pub Date : 2020-10-17 , DOI: 10.1002/hbm.25233 Paul Muhle 1, 2 , Bendix Labeit 1, 2 , Andreas Wollbrink 2 , Inga Claus 1 , Tobias Warnecke 1 , Carsten H Wolters 2 , Joachim Gross 2 , Rainer Dziewas 1 , Sonja Suntrup-Krueger 1, 2
Human Brain Mapping ( IF 3.5 ) Pub Date : 2020-10-17 , DOI: 10.1002/hbm.25233 Paul Muhle 1, 2 , Bendix Labeit 1, 2 , Andreas Wollbrink 2 , Inga Claus 1 , Tobias Warnecke 1 , Carsten H Wolters 2 , Joachim Gross 2 , Rainer Dziewas 1 , Sonja Suntrup-Krueger 1, 2
Affiliation
|
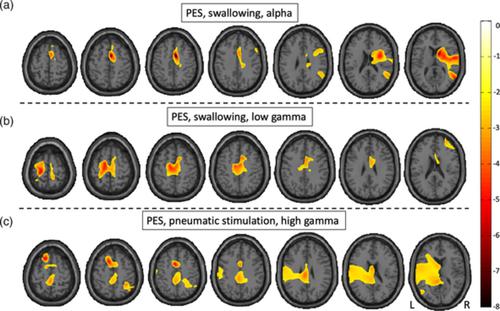
Pharyngolaryngeal hypesthesia is a major reason for dysphagia in various neurological diseases. Emerging neuromodulation devices have shown potential to foster dysphagia rehabilitation, but the optimal treatment strategy is unknown. Because functional imaging studies are difficult to conduct in severely ill patients, we induced a virtual sensory lesion in healthy volunteers and evaluated the effects of central and peripheral neurostimulation techniques. In a sham‐controlled intervention study with crossover design on 10 participants, we tested the potential of (peripheral) pharyngeal electrical stimulation (PES) and (central) transcranial direct current stimulation (tDCS) to revert the effects of lidocaine‐induced pharyngolaryngeal hypesthesia on central sensorimotor processing. Changes were observed during pharyngeal air‐pulse stimulation and voluntary swallowing applying magnetoencephalography before and after the interventions. PES induced a significant (p < .05) increase of activation during swallowing in the bihemispheric sensorimotor network in alpha and low gamma frequency ranges, peaking in the right premotor and left primary sensory area, respectively. With pneumatic stimulation, significant activation increase was found after PES in high gamma peaking in the left premotor area. Significant changes of brain activation after tDCS could neither be detected for pneumatic stimulation nor for swallowing. Due to the peripheral cause of dysphagia in this model, PES was able to revert the detrimental effects of reduced sensory input on central processing, whereas tDCS was not. Results may have implications for therapeutic decisions in the clinical context.
中文翻译:

针对吞咽网络内的感觉反馈——通过中枢和外周刺激策略逆转人工诱发的咽喉感觉迟钝
咽喉感觉迟钝是各种神经系统疾病吞咽困难的主要原因。新兴的神经调节装置已显示出促进吞咽困难康复的潜力,但最佳治疗策略尚不清楚。由于功能成像研究难以在重症患者中进行,我们在健康志愿者中诱导了虚拟感觉损伤,并评估了中枢和外周神经刺激技术的效果。在一项对 10 名参与者进行交叉设计的假控制干预研究中,我们测试了(外周)咽电刺激(PES)和(中央)经颅直流电刺激(tDCS)恢复利多卡因引起的咽喉感觉迟钝对中央感觉运动处理。在干预前后应用脑磁图在咽部空气脉冲刺激和自愿吞咽期间观察到变化。PES 诱导显着 (p < .05) 在 alpha 和低 gamma 频率范围内吞咽过程中双半球感觉运动网络的激活增加,分别在右侧运动前区和左侧初级感觉区域达到峰值。通过气动刺激,PES 后左侧运动前区的高伽马峰值出现显着激活增加。tDCS 后脑激活的显着变化既不能用于气动刺激也不能用于吞咽。由于该模型中吞咽困难的外围原因,PES 能够恢复感觉输入减少对中央处理的不利影响,而 tDCS 则不能。结果可能对临床环境中的治疗决策产生影响。
更新日期:2020-10-17
中文翻译:

针对吞咽网络内的感觉反馈——通过中枢和外周刺激策略逆转人工诱发的咽喉感觉迟钝
咽喉感觉迟钝是各种神经系统疾病吞咽困难的主要原因。新兴的神经调节装置已显示出促进吞咽困难康复的潜力,但最佳治疗策略尚不清楚。由于功能成像研究难以在重症患者中进行,我们在健康志愿者中诱导了虚拟感觉损伤,并评估了中枢和外周神经刺激技术的效果。在一项对 10 名参与者进行交叉设计的假控制干预研究中,我们测试了(外周)咽电刺激(PES)和(中央)经颅直流电刺激(tDCS)恢复利多卡因引起的咽喉感觉迟钝对中央感觉运动处理。在干预前后应用脑磁图在咽部空气脉冲刺激和自愿吞咽期间观察到变化。PES 诱导显着 (p < .05) 在 alpha 和低 gamma 频率范围内吞咽过程中双半球感觉运动网络的激活增加,分别在右侧运动前区和左侧初级感觉区域达到峰值。通过气动刺激,PES 后左侧运动前区的高伽马峰值出现显着激活增加。tDCS 后脑激活的显着变化既不能用于气动刺激也不能用于吞咽。由于该模型中吞咽困难的外围原因,PES 能够恢复感觉输入减少对中央处理的不利影响,而 tDCS 则不能。结果可能对临床环境中的治疗决策产生影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号