当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Reduction of Carbon Dioxide and Iron Oxide in Molten Salts to Fe/Fe3C Modified Carbon for Electrocatalytic Oxygen Evolution
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-16 , DOI: 10.1002/anie.202013257 Xinxin Liang 1 , Juanxiu Xiao 2 , Wei Weng 1, 3 , Wei Xiao 1, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-16 , DOI: 10.1002/anie.202013257 Xinxin Liang 1 , Juanxiu Xiao 2 , Wei Weng 1, 3 , Wei Xiao 1, 3
Affiliation
|
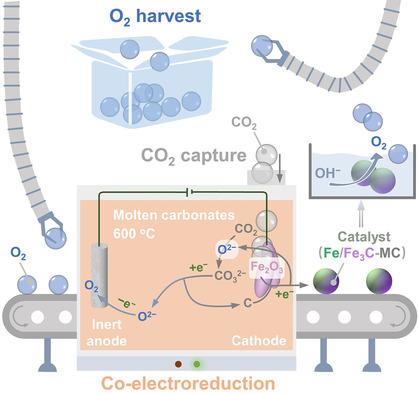
Non‐noble electrocatalyst for the oxygen evolution reaction (OER) is essential for water electrolysis and electrochemical conversion of CO2. Integrating electrochemical fixation of CO2 and electrochemical metallurgy to prepare advanced OER electrocatalyst is a promising solution to promote carbon neutrality and renewable energy. Herein, the electrochemical reduction of CO2 and Fe2O3 are combined in molten salts to prepare cathodic Fe3C‐based electrocatalyst and anodic oxygen at 600 °C with enhanced current efficiency. The resulting Fe3C‐based electrocatalyst outperforms precious electrocatalyst towards the OER operation in 1 M KOH due to a dynamic structural evolution to form an interface of Fe3C‐FeOOH.
中文翻译:

熔融盐中的二氧化碳和氧化铁电化学还原为Fe / Fe3C修饰的碳,用于电催化析氧
用于氧气析出反应(OER)的非贵金属电催化剂对于水电解和CO 2的电化学转化至关重要。将CO 2的电化学固定与电化学冶金相结合以制备高级OER电催化剂是促进碳中和和可再生能源的有前途的解决方案。在这里,将CO 2和Fe 2 O 3的电化学还原结合在熔融盐中,以提高的电流效率在600°C下制备基于Fe 3 C的阴极电催化剂和阳极氧。生成的Fe 3由于动态结构演变形成了Fe 3 C-FeOOH的界面,因此基于C的电催化剂在1 M KOH中比OER操作的性能要好于珍贵的电催化剂。
更新日期:2020-10-16
中文翻译:

熔融盐中的二氧化碳和氧化铁电化学还原为Fe / Fe3C修饰的碳,用于电催化析氧
用于氧气析出反应(OER)的非贵金属电催化剂对于水电解和CO 2的电化学转化至关重要。将CO 2的电化学固定与电化学冶金相结合以制备高级OER电催化剂是促进碳中和和可再生能源的有前途的解决方案。在这里,将CO 2和Fe 2 O 3的电化学还原结合在熔融盐中,以提高的电流效率在600°C下制备基于Fe 3 C的阴极电催化剂和阳极氧。生成的Fe 3由于动态结构演变形成了Fe 3 C-FeOOH的界面,因此基于C的电催化剂在1 M KOH中比OER操作的性能要好于珍贵的电催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号