当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Utilization of Lanthipeptide Synthetases Is a General Strategy for the Biosynthesis of 2‐Aminovinyl‐Cysteine Motifs in Thioamitides**
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-10-16 , DOI: 10.1002/anie.202012871 Jingxia Lu 1 , Yuan Wu 1 , Yuqing Li 1 , Huan Wang 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-10-16 , DOI: 10.1002/anie.202012871 Jingxia Lu 1 , Yuan Wu 1 , Yuqing Li 1 , Huan Wang 1
Affiliation
|
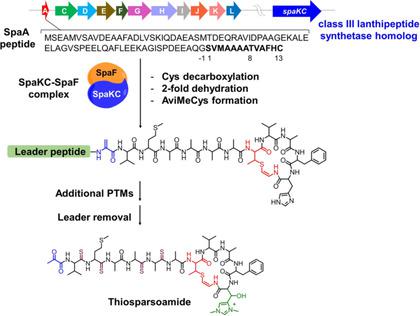
The biosynthesis of thioamitide natural products remains largely unknown, especially for the characteristic C‐terminal 2‐aminovinyl‐cysteine (AviCys) motifs. Herein, we report the discovery that homologues of class‐III lanthipeptide synthetases (LanKCts) encoded outside putative thioamitide biosynthetic gene clusters (BGCs) fully dehydrate the precursor peptides. LanKCt enzymes bind tightly to cysteine decarboxylases encoded inside thioamitide BGCs and the resulting enzyme complex completes the macrocyclization of AviCys rings. Furthermore, LanKCt enzymes are present in the genomes of many thioamitide‐producing strains and participate in the generation of AviCys macrocycles. Together, our study reveals an unprecedented system that lanthipeptide synthetases outside thioamitide BGCs participate in their biosynthesis by specific association with cysteine decarboxylases encoded inside BGCs.
中文翻译:

利用硫肽合成酶是硫代酰胺中2-氨基乙烯基-半胱氨酸基序生物合成的一般策略**
硫代氨基化物天然产物的生物合成仍然非常未知,特别是对于特征性的C末端2-氨基乙烯基-半胱氨酸(AviCys)图案。在这里,我们报告发现发现,在推定的硫代氨基甲酰胺生物合成基因簇(BGC)外部编码的III类脂肽合成酶(LanKC t s)的同源物使前体肽完全脱水。LanKCt酶与硫代酰胺BGC内部编码的半胱氨酸脱羧酶紧密结合,所得酶复合物完成了AviCys环的大环化。此外,LanKC t这种酶存在于许多产生硫代酰胺的菌株的基因组中,并参与AviCys大环的产生。在一起,我们的研究揭示了一种前所未有的系统,即硫代酰胺BGC之外的肽肽合成通过与BGC内部编码的半胱氨酸脱羧酶特异性结合而参与其生物合成。
更新日期:2020-10-16
中文翻译:

利用硫肽合成酶是硫代酰胺中2-氨基乙烯基-半胱氨酸基序生物合成的一般策略**
硫代氨基化物天然产物的生物合成仍然非常未知,特别是对于特征性的C末端2-氨基乙烯基-半胱氨酸(AviCys)图案。在这里,我们报告发现发现,在推定的硫代氨基甲酰胺生物合成基因簇(BGC)外部编码的III类脂肽合成酶(LanKC t s)的同源物使前体肽完全脱水。LanKCt酶与硫代酰胺BGC内部编码的半胱氨酸脱羧酶紧密结合,所得酶复合物完成了AviCys环的大环化。此外,LanKC t这种酶存在于许多产生硫代酰胺的菌株的基因组中,并参与AviCys大环的产生。在一起,我们的研究揭示了一种前所未有的系统,即硫代酰胺BGC之外的肽肽合成通过与BGC内部编码的半胱氨酸脱羧酶特异性结合而参与其生物合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号