Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2020-10-17 , DOI: 10.1016/j.jcmgh.2020.10.006 Linqiang Zhang 1 , Zhiguo Zhang 2 , Chengbin Li 3 , Tingting Zhu 2 , Jing Gao 4 , Hu Zhou 4 , Yingzhuan Zheng 5 , Qing Chang 6 , Mingshan Wang 7 , Jieyu Wu 2 , Liyuan Ran 8 , Yingjie Wu 9 , Huilai Miao 10 , Xiaoju Zou 11 , Bin Liang 12
|
Background & Aims
Nonalcoholic fatty liver disease (NAFLD) is becoming a severe liver disorder worldwide. Autophagy plays a critical role in liver steatosis. However, the role of autophagy in NAFLD remains exclusive and under debate. In this study, we investigated the role of S100 calcium binding protein A11 (S100A11) in the pathogenesis of hepatic steatosis.
Methods
We performed liver proteomics in a well-established tree shrew model of NAFLD. The expression of S100A11 in different models of NAFLD was detected by Western blot and/or quantitative polymerase chain reaction. Liver S100A11 overexpression mice were generated by injecting a recombinant adenovirus gene transfer vector through the tail vein and then induced by a high-fat and high-cholesterol diet. Cell lines with S100a11 stable overexpression were established with a recombinant lentiviral vector. The lipid content was measured with either Bodipy staining, Oil Red O staining, gas chromatography, or a triglyceride kit. The autophagy and lipogenesis were detected in vitro and in vivo by Western blot and quantitative polymerase chain reaction. The functions of Sirtuin 1, histone deacetylase 6 (HDAC6), and FOXO1 were inhibited by specific inhibitors. The interactions between related proteins were analyzed by a co-immunoprecipitation assay and immunofluorescence analysis.
Results
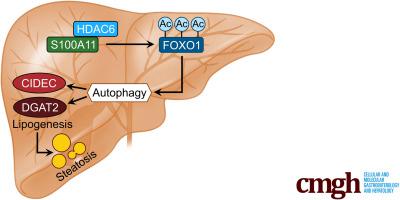
The expression of S100A11 was up-regulated significantly in a time-dependent manner in the tree shrew model of NAFLD. S100A11 expression was induced consistently in oleic acid–treated liver cells as well as the livers of mice fed a high-fat diet and NAFLD patients. Both in vitro and in vivo overexpression of S100A11 could induce hepatic lipid accumulation. Mechanistically, overexpression of S100A11 activated an autophagy and lipogenesis process through up-regulation and acetylation of the transcriptional factor FOXO1, consequently promoting lipogenesis and lipid accumulation in vitro and in vivo. Inhibition of HDAC6, a deacetylase of FOXO1, showed similar phenotypes to S100A11 overexpression in Hepa 1–6 cells. S100A11 interacted with HDAC6 to inhibit its activity, leading to the release and activation of FOXO1. Under S100A11 overexpression, the inhibition of FOXO1 and autophagy could alleviate the activated autophagy as well as up-regulated lipogenic genes. Both FOXO1 and autophagy inhibition and Dgat2 deletion could reduce liver cell lipid accumulation significantly.
Conclusions
A high-fat diet promotes liver S100A11 expression, which may interact with HDAC6 to block its binding to FOXO1, releasing or increasing the acetylation of FOXO1, thus activating autophagy and lipogenesis, and accelerating lipid accumulation and liver steatosis. These findings indicate a completely novel S100A11-HDAC6-FOXO1 axis in the regulation of autophagy and liver steatosis, providing potential possibilities for the treatment of NAFLD.
中文翻译:

S100A11 通过 FOXO1 介导的自噬和脂肪生成促进肝脂肪变性
背景与目标
非酒精性脂肪性肝病 (NAFLD) 正在成为世界范围内严重的肝脏疾病。自噬在肝脂肪变性中起关键作用。然而,自噬在 NAFLD 中的作用仍然是排他性的并且存在争议。在这项研究中,我们研究了 S100 钙结合蛋白 A11 (S100A11) 在肝脂肪变性发病机制中的作用。
方法
我们在成熟的 NAFLD 树鼩模型中进行了肝脏蛋白质组学。Western blot和/或定量聚合酶链反应检测S100A11在不同NAFLD模型中的表达。通过尾静脉注射重组腺病毒基因转移载体,然后通过高脂肪和高胆固醇饮食诱导,产生肝脏 S100A11 过表达小鼠。带有S100a11 的细胞系用重组慢病毒载体建立稳定的过表达。脂质含量用 Bodipy 染色、油红 O 染色、气相色谱法或甘油三酯试剂盒测量。通过蛋白质印迹和定量聚合酶链反应在体外和体内检测自噬和脂肪生成。Sirtuin 1、组蛋白去乙酰化酶 6 (HDAC6) 和 FOXO1 的功能受到特定抑制剂的抑制。通过免疫共沉淀分析和免疫荧光分析分析相关蛋白质之间的相互作用。
结果
S100A11的表达在NAFLD树鼩模型中呈时间依赖性显着上调。S100A11 表达在油酸处理的肝细胞以及喂食高脂肪饮食的小鼠和 NAFLD 患者的肝脏中持续诱导。S100A11 的体外和体内过表达均可诱导肝脏脂质积累。从机制上讲,S100A11 的过表达通过转录因子 FOXO1 的上调和乙酰化激活了自噬和脂肪生成过程,从而促进了体外和体内的脂肪生成和脂质积累。HDAC6(FOXO1 的一种脱乙酰酶)的抑制显示出与 Hepa 1-6 细胞中 S100A11 过表达相似的表型。S100A11 与 HDAC6 相互作用以抑制其活性,导致 FOXO1 的释放和激活。在 S100A11 过表达下,FOXO1和自噬的抑制可以减轻激活的自噬以及上调脂肪生成基因。FOXO1 和自噬抑制以及 Dgat2 缺失都可以显着减少肝细胞脂质积累。
结论
高脂饮食会促进肝脏 S100A11 的表达,它可能与 HDAC6 相互作用,阻断其与 FOXO1 的结合,释放或增加 FOXO1 的乙酰化,从而激活自噬和脂肪生成,加速脂质积累和肝脂肪变性。这些发现表明一个全新的 S100A11-HDAC6-FOXO1 轴在自噬和肝脏脂肪变性的调节中,为 NAFLD 的治疗提供了潜在的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号