Neurobiology of Learning and Memory ( IF 2.2 ) Pub Date : 2020-10-17 , DOI: 10.1016/j.nlm.2020.107327 Amy F T Arnsten 1
|
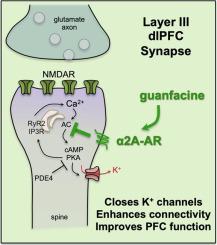
The selective norepinephrine (NE) α2A-adrenoceptor (α2A-AR) agonist, guanfacine (Intuniv™), is FDA-approved for treating Attention Deficit Hyperactivity Disorder (ADHD) based on research in animals, a translational success story. Guanfacine is also widely used off-label in additional mental disorders that involve impaired functioning of the prefrontal cortex (PFC), including stress-related disorders such as substance abuse, schizotypic cognitive deficits, and traumatic brain injury. The PFC subserves high order cognitive and executive functions including working memory, abstract reasoning, insight and judgment, and top-down control of attention, action and emotion. These abilities arise from PFC microcircuits with extensive recurrent excitation through NMDAR synapses. There is powerful modulation of these synapses, where cAMP-PKA opening of nearby potassium (K+) channels can rapidly and dynamically alter synaptic strength to coordinate arousal state with cognitive state, e.g. to take PFC “offline” during uncontrollable stress. A variety of evidence shows that guanfacine acts within the PFC via post-synaptic α2A-AR on dendritic spines to inhibit cAMP-PKA-K+ channel signaling, thus strengthening network connectivity, enhancing PFC neuronal firing, and improving PFC cognitive functions. Although guanfacine’s beneficial effects are present in rodent, they are especially evident in primates, where the PFC greatly expands and differentiates. In addition to therapeutic actions in PFC, stress-related disorders may also benefit from additional α2-AR actions, such as weakening plasticity in the amygdala, reducing NE release, and anti-inflammatory actions by deactivating microglia. Altogether, these NE α2-AR actions optimize top-down control by PFC networks, which may explain guanfacine’s benefits in a variety of mental disorders.
中文翻译:

胍法辛治疗前额皮质疾病的作用机制:跨物种的成功转化
选择性去甲肾上腺素 (NE) α2A-肾上腺素受体 (α2A-AR) 激动剂胍法辛 (Intuniv™) 经 FDA 批准用于治疗注意力缺陷多动障碍 (ADHD),这是基于动物研究的转化成功案例。胍法辛还广泛用于超说明书治疗涉及前额皮质 (PFC) 功能受损的其他精神疾病,包括与压力相关的疾病,如药物滥用、精神分裂性认知缺陷和创伤性脑损伤。 PFC 促进高阶认知和执行功能,包括工作记忆、抽象推理、洞察力和判断力,以及对注意力、行动和情感的自上而下的控制。这些能力源自 PFC 微电路,通过 NMDAR 突触进行广泛的循环激励。这些突触存在强大的调节作用,其中附近钾 (K + ) 通道的 cAMP-PKA 打开可以快速、动态地改变突触强度,以协调唤醒状态与认知状态,例如在无法控制的压力期间使 PFC“离线”。多种证据表明,胍法辛在 PFC 内通过树突棘上的突触后 α2A-AR 发挥作用,抑制 cAMP-PKA-K +通道信号传导,从而增强网络连接、增强 PFC 神经元放电并改善 PFC 认知功能。尽管胍法辛的有益作用在啮齿动物中也存在,但在灵长类动物中尤其明显,因为灵长类动物的 PFC 大大扩展和分化。除了 PFC 的治疗作用外,应激相关疾病也可能受益于额外的 α2-AR 作用,例如削弱杏仁核的可塑性、减少 NE 的释放以及通过灭活小胶质细胞来发挥抗炎作用。 总而言之,这些 NE α2-AR 作用优化了 PFC 网络自上而下的控制,这可能解释了胍法辛在多种精神障碍中的益处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号