Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-10-16 , DOI: 10.1016/j.jsb.2020.107649 Silke Kleinboelting 1 , Jonas Miehling 2 , Clemens Steegborn 3
|
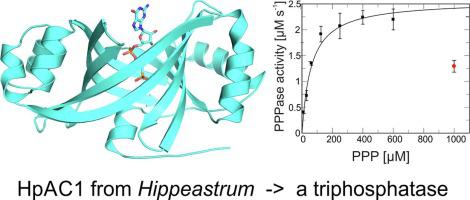
HpAC1, a protein from Hippeastrum hybrid cultivars, was previously suggested to be a plant adenylyl cyclase. We describe a structural and enzymatic characterization of HpAC1. A crystal structure of HpAC1 in complex with a non-hydrolyzable GTP analog confirms a generic CYTH architecture, comprising a β-barrel with an internal substrate site. The structure reveals significant active site differences to AC proteins with CYTH fold, however, and we find that HpAC1 lacks measurable AC activity. Instead, HpAC1 has substantial triphosphatase activity, indicating this protective activity or a related activity as the protein’s physiological function.
中文翻译:

来自朱顶红的推定腺苷酸环化酶 HpAC1 的晶体结构和酶学表征揭示了主要的三磷酸酶活性
HpAC1 是一种来自朱顶红杂交品种的蛋白质,以前被认为是一种植物腺苷酸环化酶。我们描述了 HpAC1 的结构和酶学特征。HpAC1 与不可水解的 GTP 类似物复合的晶体结构证实了通用的 CYTH 结构,包括具有内部底物位点的 β-桶。然而,该结构揭示了具有 CYTH 折叠的 AC 蛋白的显着活性位点差异,我们发现 HpAC1 缺乏可测量的 AC 活性。相反,HpAC1 具有显着的三磷酸酶活性,表明这种保护活性或相关活性作为蛋白质的生理功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号