Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-10-16 , DOI: 10.1016/j.jinorgbio.2020.111272 Paolo Ascenzi 1 , Giovanna De Simone 2 , Andrea Pasquadibisceglie 2 , Magda Gioia 3 , Massimo Coletta 3
|
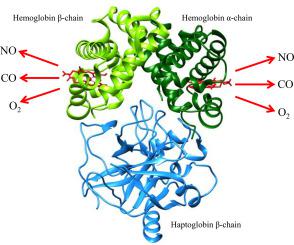
Haptoglobin (Hp) counterbalances the adverse effects of extra-erythrocytic hemoglobin (Hb) by trapping the αβ dimers of Hb in the bloodstream. In turn, the Hp:Hb complexes display Hb-like reactivity. Here, the kinetics of NO dissociation from ferrous nitrosylated Hp:Hb complexes (i.e., Hp1–1:Hb(II)-NO and Hp2–2:Hb(II)-NO, respectively) are reported at pH 7.0 and 20.0 °C. NO dissociation from Hp:Hb(II)-NO complexes has been followed by replacing NO with CO. Denitrosylation kinetics of Hp1–1:Hb(II)-NO and Hp2–2:Hb(II)-NO are biphasic, the relative amplitude of the fast and slow phase being 0.495 ± 0.015 and 0.485 ± 0.025, respectively. Values of koff(NO)1 and koff(NO)2 (i.e., (6.4 ± 0.8) × 10−5 s−1 and (3.6 ± 0.6) × 10−5 s−1 for Hp1–1:Hb(II)-NO and (5.8 ± 0.8) × 10−5 s−1 and (3.1 ± 0.6) × 10−5 s−1 for Hp2–2:Hb(II)-NO) are unaffected by allosteric effectors and correspond to those reported for the α and β subunits of tetrameric Hb(II)-NO and isolated α(II)-NO and β(II)-NO chains, respectively. This highlights the view that the conformation of the Hb α1β1 and α2β2 dimers matches that of the Hb high affinity conformation. Moreover, the observed functional heterogeneity reflects the variation of energy barriers for the ligand detachment and exit pathway(s) associated to the different structural arrangement of the two subunits in the nitrosylated R-state. Noteworthy, the extent of the inequivalence of α and β chains is closely similar for the O2, NO and CO dissociation in the R-state, suggesting that it is solely determined by the structural difference between the two subunits.
中文翻译:

配体从亚硝基化人触珠蛋白:血红蛋白复合物解离的 α 和 β 亚基之间的动力学不等价。与 O2 和 CO 解离的比较
触珠蛋白 (Hp) 通过在血流中捕获 Hb 的 αβ 二聚体来抵消红细胞外血红蛋白 (Hb) 的不利影响。反过来,Hp:Hb 复合物显示出类似 Hb 的反应性。在这里,亚铁基化 Hp:Hb 复合物(即分别为Hp1-1:Hb(II)-NO 和 Hp2-2:Hb(II)-NO)的 NO 解离动力学在 pH 7.0 和 20.0 °C 下报告. NO 从 Hp:Hb(II)-NO 复合物解离之后,用 CO 替换 NO。Hp1-1:Hb(II)-NO 和 Hp2-2:Hb(II)-NO 的脱亚硝基化动力学是双相的,相对的快慢相的幅值分别为 0.495±0.015 和 0.485±0.025。的值ķ断开(NO)1和ķ关闭(NO)2(即,(6.4±0.8)×10 -5 s -1和 (3.6 ± 0.6) × 10 -5 s -1对于 Hp1–1:Hb(II)-NO 和 (5.8 ± 0.8) × 10 -5 s -1和 (3.1 ± 0.6) × 10 -5 Hp2–2:Hb(II)-NO) 的 s -1不受变构效应物的影响,对应于四聚体 Hb(II)-NO 的 α 和 β 亚基以及分离的 α(II)-NO 和 β( II)-NO 链,分别。这突出了 Hb α 1 β 1和 α 2 β 2构象的观点二聚体与 Hb 高亲和力构象相匹配。此外,观察到的功能异质性反映了与亚硝基化 R 状态中两个亚基的不同结构排列相关的配体脱离和退出途径的能量障碍的变化。值得注意的是,α 和 β 链的不等价程度对于 R 态中的 O 2、NO 和 CO 解离非常相似,这表明它完全由两个亚基之间的结构差异决定。



























 京公网安备 11010802027423号
京公网安备 11010802027423号