Insect Biochemistry and Molecular Biology ( IF 3.2 ) Pub Date : 2020-10-17 , DOI: 10.1016/j.ibmb.2020.103488 André C. Pimentel , Renata O. Dias , Thaís D. Bifano , Fernando A. Genta , Clelia Ferreira , Walter R. Terra
|
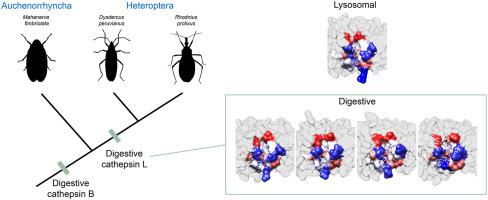
Cysteine peptidases (CP) play a role as digestive enzymes in hemipterans similar to serine peptidases in most other insects. There are two major CPs: cathepsin L (CAL), which is an endopeptidase and cathepsin B (CAB) that is both an exopeptidase and a minor endopeptidase. There are thirteen putative CALs in Dysdercus peruvianus, which in some cases were confirmed by cloning their encoding genes. RNA-seq data showed that DpCAL5 is mainly expressed in the anterior midgut (AM), DpCAL10 in carcass (whole body less midgut), suggesting it is a lysosomal enzyme, and the other DpCALs are expressed in middle (MM) and posterior (PM) midgut. The expression data were confirmed by qPCR and enzyme secretion to midgut lumen by a proteomic approach. Two CAL activities were isolated by chromatography from midgut samples with similar kinetic properties toward small substrates. Docking analysis of a long peptide with several DpCALs modeled with digestive Tenebrio molitor CAL (TmCAL3) as template showed that on adapting to luminal digestion DpCALs (chiefly DpCAL5) changed in relation to their ancestral lysosomal enzyme (DpCAL10) mainly at its S2 subsite. A similar conclusion arrived from structure alignment-based clustering of DpCALs based on structural similarity of the modeled structures. Changes mostly on S2 subsite could mean the enzymes turn out less peptide-bond selective, as described in TmCALs. R. prolixus CALs changed on adapting to luminal digestion, although less than DpCALs. Both D. peruvianus and R. prolixus have two digestive CABs which are expressed in the same extension as CALs, in the first digestive section of the midgut, but less than in the other midgut sections. Mahanarva fimbriolata does not seem to have digestive CALs and their digestive CABs are mainly expressed in the first digestive section of the midgut and do not diverge much from their lysosomal counterparts. The data suggest that CABs are necessary at the initial stage of digestion in CP-dependent Hemipterans, which action is completed by CALs with low peptide-bond selectivity in Heteroptera species. In M. fimbriolata protein digestion is supposed to be associated with the inactivation of sap noxious proteins, making CAB sufficient as digestive CP. Hemipteran genomes and transcriptome data showed that CALs have been recruited as digestive enzymes only in heteropterans, whereas digestive CABs occur in all hemipterans.
中文翻译:

Dysdercus peruvianus,Rhodnius prolixus和Mahanarva fimbriolata中的组织蛋白酶L和B。寻找适合消化的酶
半胱氨酸肽酶(CP)在半足动物中起消化酶的作用,与大多数其他昆虫中的丝氨酸肽酶相似。有两种主要的CP:组织蛋白酶L(CAL)(一种内肽酶)和组织蛋白酶B(CAB)(一种外肽酶和次要的内肽酶)。秘鲁dysdercus peruvianus有13个推定的CAL,在某些情况下可通过克隆其编码基因得到确认。RNA-seq数据显示DpCAL5主要在前中肠(AM)中表达,DpCAL10在car体中(全身少中肠),表明它是一种溶酶体酶,其他DpCALs在中部(MM)和后部(PM)表达)中肠。通过qPCR和通过蛋白质组学方法将酶分泌至中肠腔证实了表达数据。通过色谱法从中肠样品中分离出两种CAL活性,这些样品对小底物具有相似的动力学特性。用消化性黄粉虫模拟的几种DpCAL对长肽的对接分析CAL(TmCAL3)作为模板显示,DpCAL(主要是DpCAL5)适应腔消化后,其祖先溶酶体酶(DpCAL10)主要在其S2亚位发生了变化。基于建模结构的结构相似性,基于结构对齐的DpCAL聚类得出相似的结论。如TmCAL中所述,大部分在S2亚位点的变化可能意味着这些酶对肽键的选择性降低。尽管比DpCAL少,但R. prolixus CAL在适应腔消化方面发生了变化。既D. peruvianus和R.蝽具有被在相同的延伸的CAL中表达,在中肠中的第一消化第二节消化驾驶室,但小于在其他肠部分。毛han似乎没有消化CAL,其消化CAB主要在中肠的第一个消化区表达,与溶酶体对应物的差异不大。数据表明,在依赖CP的半翅目动物消化的初始阶段,CAB是必需的,而这一作用是由异翅目物种中具有低肽键选择性的CAL完成的。在纤毛分枝杆菌中,蛋白质的消化被认为与树液中有害蛋白质的失活有关,使得CAB足以作为消化性CP。半足动物的基因组和转录组数据显示,CAL仅在异足动物中被招募为消化酶,而消化CABs在所有半足动物中都存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号