Catalysis Today ( IF 5.2 ) Pub Date : 2020-10-16 , DOI: 10.1016/j.cattod.2020.10.005 Julián A. Vannucci , Nora N. Nichio , Francisco Pompeo
|
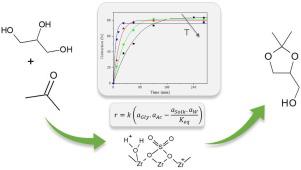
In this work, a series of acid catalysts were synthetized from a commercial zirconium oxide sulfated with a 0.5 M H2SO4 solution by wet impregnation. The characterization results show a correlation between the calcination temperature and the acid sites generated on the materials. Among the catalysts prepared, the sulfated zirconia calcined in air at 400 °C (Zr-S-400), with a molar ratio S/Zr = 0.23 was the most active one due to its larger acid density and greater acid strength caused by the generation of new Brönsted sites. The Zr-S-400 catalyst exhibited an initial reaction rate of 0.0497 mol.min-1. g-1, and achieved a glycerol conversion of 80% in 1 hour of reaction at 40 °C (glycerol:acetone molar ratio = 1:6). The Zr-S-400 material remained stable after four catalytic cycles, demonstrating the stability of the superficial sulfate species (S/Zr ∼ 0.2). In addition, the thermodynamics and kinetics of the reaction were evaluated, as well as the influence of some operating conditions such as the molar ratio of reactants and the water content in the reaction mixture. The following standard molar reaction properties were obtained: ΔHº = -11.6 ± 1.1 kJ.mol-1 and ΔGº = 4.0 ± 0.1 kJ.mol-1. Taking into account that the adsorption of water on this catalyst did not affect the number of acid sites available, a simple pseudo-homogeneous kinetic expression was developed and successfully adjusted to the experimental data in the range under study. Based on this model, the estimated activation energy of the reaction was 88.1 ± 8.9 kJ.mol-1.
中文翻译:

甘油与丙酮的缩酮化合成缩酮:硫酸化氧化锆催化剂的动力学研究
在这项工作中,通过湿浸渍从用0.5 MH 2 SO 4溶液硫酸化的市售氧化锆合成了一系列酸催化剂。表征结果显示了煅烧温度与材料上产生的酸位之间的相关性。在所制得的催化剂中,在400°C(Zr-S-400)的空气中煅烧的硫酸化氧化锆(Sr / Zr = 0.23)是最活泼的催化剂,因为它的酸密度更大,且酸强度更高。产生新的布朗斯台德遗址。Zr-S-400催化剂的初始反应速率为0.0497mol.min -1。g -1,在40°C(甘油:丙酮摩尔比= 1:6)下反应1小时,甘油转化率达到80%。Zr-S-400材料在四个催化循环后保持稳定,证明了表面硫酸盐的稳定性(S / Zr约为0.2)。另外,评估了反应的热力学和动力学,以及一些操作条件的影响,例如反应物的摩尔比和反应混合物中的水含量。获得以下标准摩尔反应性质:ΔHº= -11.6±1.1 kJ.mol -1和ΔGº= 4.0±0.1 kJ.mol -1。考虑到水在该催化剂上的吸附不会影响可用酸位的数量,因此开发了一种简单的假均相动力学表达式,并成功地将其调整为所研究范围内的实验数据。根据该模型,估计的反应活化能为88.1±8.9 kJ.mol -1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号