Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-10-16 , DOI: 10.1016/j.bbagen.2020.129765 Anindita Roy , Yuma Miyai , Alessandro Rossi , Krishna Paraswar , Umesh R. Desai , Yukio Saijoh , Balagurunathan Kuberan
|
Background
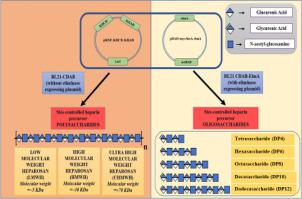
Heparin, a lifesaving blood thinner used in over 100 million surgical procedures worldwide annually, is currently isolated from over 700 million pigs and ~200 million cattle in slaughterhouses worldwide. Though animal-derived heparin has been in use over eight decades, it is a complex mixture that poses a risk for chemical adulteration, and its availability is highly vulnerable. Therefore, there is an urgent need in devising bioengineering approaches for the production of heparin polymers, especially low molecular weight heparin (LMWH), and thus, relying less on animal sources. One of the main challenges, however, is the rapid, cost-effective production of low molecular weight heparosan, a precursor of LMWH and size-defined heparosan oligosaccharides. Another challenge is N-sulfation of N-acetyl heparosan oligosaccharides efficiently, an essential modification required for subsequent enzymatic modifications, though chemical and enzymatic N-sulfation is effectively performed at the polymer level.
Methods
To devise a strategy to produce low molecular weight heparosan and heparosan oligosaccharides, several non-pathogenic E. coli strains are engineered by transforming the essential heparosan biosynthetic genes with or without the eliminase gene (elmA) from pathogenic E. coli K5.
Results
The metabolically engineered non-pathogenic strains are shown to produce ~5 kDa heparosan, a precursor for low molecular weight heparin, for the first time. Additionally, heparosan oligosaccharides of specific sizes ranging from tetrasaccharide to dodecasaccharide are directly generated, in a single step, from the recombinant bacterial strains that carry both heparosan biosynthetic genes and the eliminase gene. Various modifications, such as chemical N-sulfation of N-acetyl heparosan hexasaccharide following the selective protection of reducing end GlcNAc residue, enzymatic C5-epimerization of N-sulfo heparosan tetrasaccharide and complete 6-O sulfation of N-sulfo heparosan hexasaccharide, are shown to be feasible.
Conclusions
We engineered non-pathogenic E. coli strains to produce low molecular weight heparosan and a range of size-specific heparosan oligosaccharides in a controlled manner through modulating culture conditions. We have also shown various chemical and enzymatic modifications of heparosan oligosaccharides.
General significance
Heparosan is a precursor of heparin and the methods to produce low molecular weight heparosan is widely awaited. The methods described herein are promising and will pave the way for potential large scale production of low molecular weight heparin anticoagulants and bioactive heparin oligosaccharides in the coming decade.
中文翻译:

非病原性大肠杆菌菌株的代谢工程,可控制生产低分子量肝素和大小特异性肝素寡糖
背景
肝素是一种拯救生命的血液稀释剂,每年在全球超过1亿次手术中使用,目前在全球屠宰场中从7亿多头猪和约2亿头牛中分离出来。尽管动物肝素已经使用了八十多年,但是它是一种复杂的混合物,存在化学掺杂的风险,并且其可用性非常脆弱。因此,迫切需要设计生物工程方法来生产肝素聚合物,尤其是低分子量肝素(LMWH),从而减少对动物来源的依赖。然而,主要挑战之一是快速,经济高效地生产低分子量肝素,这是LMWH和尺寸确定的肝素寡糖的前体。另一个挑战是ň的-sulfation ñ-乙酰肝素低聚糖有效,这是后续酶促修饰所需的必要修饰,尽管化学和酶促N-硫酸化可在聚合物水平上有效地进行。
方法
为了设计生产低分子量肝素和肝素寡糖的策略,通过转化具有或不具有来自病原性大肠杆菌K5的消除酶基因(elmA)的必需肝素生物合成基因,设计了几种非致病性大肠杆菌菌株。
结果
的代谢工程化的非致病株被示出为产生〜 5 kDa的乙酰肝素,低分子量肝素的前体,为第一次。另外,从一步携带载有肝素生物合成基因和消除酶基因的重组细菌菌株,可直接生成从四糖到十二糖的特定大小的肝素寡糖。各种修改,如化学ñ的-sulfation Ñ以下还原末端GlcNAc残基的,酶C5-差向异构化的选择性保护-乙酰基乙酰肝素己糖Ñ磺乙酰肝素四糖和完整6- ö硫酸化Ñ硫代肝素六糖被证明是可行的。
结论
我们设计了非致病性大肠杆菌菌株,通过调节培养条件,以受控方式生产低分子量肝素和一系列大小特定的肝素寡糖。我们还显示了肝素寡糖的各种化学和酶修饰。
一般意义
肝素是肝素的前体,人们广泛期待生产低分子量肝素的方法。本文所述的方法是有前途的,并将为在未来十年中潜在的大规模生产低分子量肝素抗凝剂和生物活性肝素寡糖铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号