Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 4.3 ) Pub Date : 2020-10-17 , DOI: 10.1016/j.bbabio.2020.148330 Olivier N. Lemaire , Tristan Wagner
|
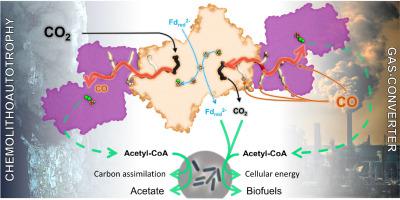
Clostridium autoethanogenum, the bacterial model for biological conversion of waste gases into biofuels, grows under extreme carbon-monoxide (CO) concentrations. The strictly anaerobic bacterium derives its entire cellular energy and carbon from this poisonous gas, therefore requiring efficient molecular machineries for CO-conversion. Here, we structurally and biochemically characterized the key enzyme of the CO-converting metabolism: the CO-dehydrogenase/Acetyl-CoA synthase (CODH/ACS). We obtained crystal structures of natively isolated complexes from fructose-grown and CO-grown C. autoethanogenum cultures. Both contain the same isoforms and if the overall structure adopts the classic α2β2 architecture, comparable to the model enzyme from Moorella thermoacetica, the ACS binds a different position on the CODH core. The structural characterization of a proteolyzed complex and the conservation of the binding interface in close homologs rejected the possibility of a crystallization artefact. Therefore, the internal CO-channeling system, critical to transfer CO generated at the C-cluster to the ACS active site, drastically differs in the complex from C. autoethanogenum. The 1.9-Å structure of the CODH alone provides an accurate picture of the new CO-routes, leading to the ACS core and reaching the surface. Increased gas accessibility would allow the simultaneous CO-oxidation and acetyl-CoA production. Biochemical experiments showed higher flexibility of the ACS subunit from C. autoethanogenum compared to M. thermoacetica, albeit monitoring similar CO-oxidation and formation rates. These results show a reshuffling of internal CO-tunnels during evolution of these Firmicutes, putatively leading to a bidirectional complex that ensure a high flux of CO-conversion toward energy conservation, acting as the main cellular powerplant.
中文翻译:

原始酶中的气体通道重排:产自产乙酸梭菌的一氧化碳脱氢酶/乙酰辅酶A合酶复合物的结构见解
自产乙醇梭菌(Clostridium autoethanogenum)是一种将废气生物转化为生物燃料的细菌模型,它在极端的一氧化碳(CO)浓度下生长。严格厌氧细菌从这种有毒气体中获取全部细胞能量和碳,因此需要有效的分子机制进行CO转化。在这里,我们在结构和生化上表征了CO转化代谢的关键酶:CO脱氢酶/乙酰辅酶A合酶(CODH / ACS)。我们从果糖种植和CO种植的C. autoethanogenum培养物中获得了天然分离的复合物的晶体结构。都包含相同的同种型,并且如果总体结构采用经典α 2 β 2架构,可比从模型酶Moorella thermoacetica,ACS在CODH核心上的位置不同。蛋白水解复合物的结构表征和紧密同源物中结合界面的保守性拒绝了结晶伪像的可能性。因此,内部CO通道系统对于将C簇中生成的CO转移到ACS活性位点至关重要,该复合物与C. autoethanogenum截然不同。仅CODH的1.9-Å结构就可以准确地描绘出新的CO路线,该路线通向ACS核心并到达表面。气体可及性的提高将允许同时发生CO氧化和乙酰CoA的生产。生化实验显示,相比于C.autoethanogenum,ACS亚单位具有更高的灵活性尽管监测相似的CO氧化和形成速率,但M. thermoacetica。这些结果表明,在这些Firmicutes的进化过程中,内部CO通道进行了改组,从而推定了双向复合物,从而确保了将CO转化为节能的高通量,从而成为主要的细胞动力装置。



























 京公网安备 11010802027423号
京公网安备 11010802027423号