当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Density Functional Theory Study on the Reducing Agents for Atomic Layer Deposition of Tungsten Using Tungsten Chloride Precursor
Applied Surface Science ( IF 6.3 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148156 Romel Hidayat , Tanzia Chowdhury , Yewon Kim , Seongyoon Kim , Tirta Rona Mayangsari , Soo-Hyun Kim , Won-Jun Lee
Applied Surface Science ( IF 6.3 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148156 Romel Hidayat , Tanzia Chowdhury , Yewon Kim , Seongyoon Kim , Tirta Rona Mayangsari , Soo-Hyun Kim , Won-Jun Lee
|
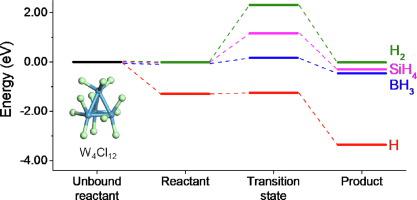
Abstract We studied co-reactants for tungsten chloride precursors by density functional theory calculation to find the proper reducing agent. Tungsten chlorides, WCl6 and WCl5, are gaining attention for the fluorine-free atomic layer deposition (ALD) of tungsten. We created a W4Cl12 cluster by optimizing the number of tungsten and chlorine atoms in the chlorine-passivated tungsten cluster. We predicted the growth of tungsten carbide by the reaction of trimethylaluminum with the cluster, confirming that the cluster can mimic the chlorine-passivated tungsten surface. Then we simulated the reaction between the W4Cl12 cluster and four co-reactants. Possible reaction pathways between the cluster and the co-reactants were simulated to compare the reaction energies and activation energies. All co-reactants of the present work, atomic hydrogen, H2, SiH4, and B2H6, would act as reducing agents with the reaction energies of −2.07 eV, −0.01 eV, −0.28 eV, and −0.45 eV, respectively. The reducing power was in the order of atomic hydrogen, B2H6, SiH4, and H2 with activation energies of +0.04 eV, +0.18 eV, +1.18 eV, and +2.32 eV, respectively. B2H6 is the most promising gas-phase candidate due to its low activation energy for reduction and high activation energy for boron incorporation.
中文翻译:

以氯化钨为前驱体制备钨原子层沉积还原剂的密度泛函理论研究
摘要 我们通过密度泛函理论计算研究了氯化钨前驱体的共反应物,以寻找合适的还原剂。氯化钨、WCl6 和 WCl5 因钨的无氟原子层沉积 (ALD) 而受到关注。我们通过优化氯钝化钨簇中钨和氯原子的数量创建了 W4Cl12 簇。我们通过三甲基铝与簇的反应预测了碳化钨的生长,证实了簇可以模拟氯钝化的钨表面。然后我们模拟了 W4Cl12 簇与四种共反应物之间的反应。模拟了簇和共反应物之间可能的反应途径,以比较反应能和活化能。当前工作的所有共反应物,原子氢,H2,SiH4,和 B2H6 将作为还原剂,反应能分别为 -2.07 eV、-0.01 eV、-0.28 eV 和 -0.45 eV。还原能力按原子氢、B2H6、SiH4 和 H2 的顺序排列,活化能分别为 +0.04 eV、+0.18 eV、+1.18 eV 和 +2.32 eV。B2H6 是最有前途的气相候选物,因为其还原活化能低,硼掺入活化能高。
更新日期:2021-02-01
中文翻译:

以氯化钨为前驱体制备钨原子层沉积还原剂的密度泛函理论研究
摘要 我们通过密度泛函理论计算研究了氯化钨前驱体的共反应物,以寻找合适的还原剂。氯化钨、WCl6 和 WCl5 因钨的无氟原子层沉积 (ALD) 而受到关注。我们通过优化氯钝化钨簇中钨和氯原子的数量创建了 W4Cl12 簇。我们通过三甲基铝与簇的反应预测了碳化钨的生长,证实了簇可以模拟氯钝化的钨表面。然后我们模拟了 W4Cl12 簇与四种共反应物之间的反应。模拟了簇和共反应物之间可能的反应途径,以比较反应能和活化能。当前工作的所有共反应物,原子氢,H2,SiH4,和 B2H6 将作为还原剂,反应能分别为 -2.07 eV、-0.01 eV、-0.28 eV 和 -0.45 eV。还原能力按原子氢、B2H6、SiH4 和 H2 的顺序排列,活化能分别为 +0.04 eV、+0.18 eV、+1.18 eV 和 +2.32 eV。B2H6 是最有前途的气相候选物,因为其还原活化能低,硼掺入活化能高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号