当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water–gas shift reaction co-catalyzed by polyoxometalate (POM)–gold composites: the “magic” role of POMs
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-10-15 , DOI: 10.1039/d0cy01722a Zhongling Lang 1, 2, 3, 4, 5 , Yangguang Li 1, 2, 3, 4, 5 , Anna Clotet 6, 7, 8, 9 , Josep M. Poblet 6, 7, 8, 9
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-10-15 , DOI: 10.1039/d0cy01722a Zhongling Lang 1, 2, 3, 4, 5 , Yangguang Li 1, 2, 3, 4, 5 , Anna Clotet 6, 7, 8, 9 , Josep M. Poblet 6, 7, 8, 9
Affiliation
|
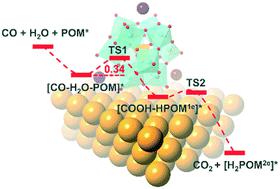
The water–gas shift reaction (WGSR, CO + H2O ↔ CO2 + H2) is an industrially important process that has been used to achieve high conversions of CO to CO2 for application in proton-exchange membrane fuel cells. Many oxide-supported gold catalysts are identified as effective catalysts for the low-temperature WGSR, but the origin of the unique catalytic properties and the role of the Au/oxide interface in this process remain under debate. In the present work, ab initio density functional theory (DFT) calculations combined with a periodic continuum solvation model were applied to provide a mechanistic network of WGSR co-catalyzed by Au(111) and polyoxometalates (POM = [PMo12O40]3− and [PW12O40]3−) in aqueous solution. The contributions of Mo(d) and O(sp) bands near the Fermi level (EF) of PMo12–Au(111) were found to be responsible for the high activity of the PMo12 modified gold catalyst, by serving as both an electron shuttle and a proton acceptor. We proposed a simple route where CO could assist water dissociation to directly form COOHads on POM–Au(111) (POMads + COads + H2Oads → COOHads + HPOMe), with barriers lower than 8 kcal mol−1 for the rate-determining step. Fully consistent with the electronic trends (W(d) > Mo(d) above EF), the PW12–Au(111) system is computed to be less active than the homologous phosphomolybdate due to the decreased basicity of O sites and lower reduction ability of W(d) orbitals. The functional mechanism and the role of POMs described in this work could inspire the design of new active WGSR catalysts.
中文翻译:

多金属氧酸盐(POM)-金复合物共同催化的水煤气变换反应:POM的“神奇”作用
水煤气变换反应(WGSR,CO + H 2 O↔CO 2 + H 2)是工业上重要的过程,已用于实现将CO转化为CO 2的高转化率,用于质子交换膜燃料电池。许多氧化物负载的金催化剂被认为是低温WGSR的有效催化剂,但是独特的催化性能的起源以及Au /氧化物界面在该过程中的作用仍在争论中。在目前的工作中,从头算密度泛函理论(DFT)计算与周期性连续溶剂化模型相结合,提供了由Au(111)和多金属氧酸盐(POM = [PMo 12 O40 ] 3-和水溶液中的[PW 12 O 40 ] 3-。发现Mo(d)和O(sp)带在PMo 12 –Au(111)的费米能级( E F)附近的贡献与PMo 12改性金催化剂的高活性有关。电子飞梭和质子受体。我们提出了一条简单的路线,其中CO可以协助水分解,从而在POM–Au(111)上直接形成COOH广告(POM广告+ CO广告+ H 2 O广告→COOH广告+ HPOM e),对于速率确定步骤,其势垒低于8 kcal mol -1。与电子的趋势完全一致(W(d)>沫上述(d)ë ˚F)时,PW 12 -Au(111)系统被计算为比同源磷不太活跃由于的O-位点和下碱度降低W(d)轨道的还原能力。这项工作中描述的POM的功能机制和作用可能会启发新型活性WGSR催化剂的设计。
更新日期:2020-11-03
中文翻译:

多金属氧酸盐(POM)-金复合物共同催化的水煤气变换反应:POM的“神奇”作用
水煤气变换反应(WGSR,CO + H 2 O↔CO 2 + H 2)是工业上重要的过程,已用于实现将CO转化为CO 2的高转化率,用于质子交换膜燃料电池。许多氧化物负载的金催化剂被认为是低温WGSR的有效催化剂,但是独特的催化性能的起源以及Au /氧化物界面在该过程中的作用仍在争论中。在目前的工作中,从头算密度泛函理论(DFT)计算与周期性连续溶剂化模型相结合,提供了由Au(111)和多金属氧酸盐(POM = [PMo 12 O40 ] 3-和水溶液中的[PW 12 O 40 ] 3-。发现Mo(d)和O(sp)带在PMo 12 –Au(111)的费米能级( E F)附近的贡献与PMo 12改性金催化剂的高活性有关。电子飞梭和质子受体。我们提出了一条简单的路线,其中CO可以协助水分解,从而在POM–Au(111)上直接形成COOH广告(POM广告+ CO广告+ H 2 O广告→COOH广告+ HPOM e),对于速率确定步骤,其势垒低于8 kcal mol -1。与电子的趋势完全一致(W(d)>沫上述(d)ë ˚F)时,PW 12 -Au(111)系统被计算为比同源磷不太活跃由于的O-位点和下碱度降低W(d)轨道的还原能力。这项工作中描述的POM的功能机制和作用可能会启发新型活性WGSR催化剂的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号