当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Hydrophobic Tail Length Variation on Surfactant-Mediated Protein Stabilization
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-10-15 , DOI: 10.1021/acs.molpharmaceut.0c00737 Mckenna G Hanson 1 , Joshua S Katz 2 , Hua Ma 2 , Miriam Putterman 2 , Benjamin A Yezer 2 , Oliver Petermann 3 , Theresa M Reineke 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-10-15 , DOI: 10.1021/acs.molpharmaceut.0c00737 Mckenna G Hanson 1 , Joshua S Katz 2 , Hua Ma 2 , Miriam Putterman 2 , Benjamin A Yezer 2 , Oliver Petermann 3 , Theresa M Reineke 1
Affiliation
|
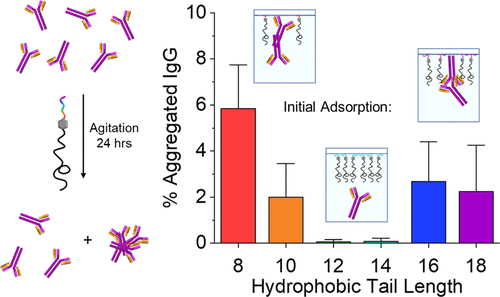
Recently, protein therapeutics have gained significant attention as a result of their enhanced selectivity and diminished side effects compared to traditional small-molecule drugs. Despite their advantages, protein formulations typically suffer from stability issues because of aggregation and denaturation during production and storage, often resulting in detrimental immune responses. Surfactants can be used to stabilize and protect proteins in solution by preventing protein adsorption onto interfaces or by forming protective structures in solution. Herein, a detailed structure–activity relationship study is described, demonstrating the role that hydrophobic tail length plays in surfactant-mediated stabilization of the model therapeutic protein IgG. The FM1000 series, originating from a surfactant scaffold that allows for easy structure modulation, was synthesized by a simple 2-step procedure. First, phenylalanine was acylated with a variety of acyl chlorides of differing lengths to yield n-acyl phenylalanine, which was then coupled to Jeffamine M1000, a polyethylene glycol-based amine, to yield the final surfactant. With this FM1000 series, it was observed that the 14 carbon-long tail surfactant (14FM1000) was optimal at preventing IgG aggregation compared to surfactants with tails that were longer or shorter. Using a combination of dynamic surface tensiometry and quartz crystal microbalance with dissipation, it was hypothesized that 14FM1000 was able to prevent IgG adsorption, and therefore aggregation, by adsorbing appreciably onto surfaces quickly. 14FM1000 had the fastest rate of initial adsorption compared to the other surfactants studied. Short-tail surfactants were slow to and did not adsorb appreciably onto surfaces, allowing IgG adsorption. Although long-tail surfactants were also slow to adsorb, allowing IgG to adsorb and aggregate, their equilibrium adsorption was strong. Additionally, 14FM1000 was the most reversibly adsorbed surfactant, likely improving its ability to desorb and adsorb quickly to transient surfaces, therefore protecting the IgG at each new hydrophobic surface and preventing aggregation. By understanding the structure–activity relationship between surfactants and protein stabilization, we move toward more efficient design of future surfactants increasing the stability and utility of important protein therapeutics.
中文翻译:

疏水性尾长变化对表面活性剂介导的蛋白质稳定的影响
最近,蛋白质疗法由于与传统小分子药物相比具有更高的选择性和更少的副作用而备受关注。尽管具有优势,但蛋白质制剂通常会因生产和储存过程中的聚集和变性而受到稳定性问题的困扰,这通常会导致有害的免疫反应。表面活性剂可用于通过防止蛋白质吸附到界面上或通过在溶液中形成保护结构来稳定和保护溶液中的蛋白质。在此,描述了详细的结构-活性关系研究,证明了疏水尾长在表面活性剂介导的模型治疗蛋白 IgG 的稳定中所起的作用。FM1000 系列源自表面活性剂支架,可轻松进行结构调制,通过简单的两步程序合成。首先,苯丙氨酸用各种不同长度的酰氯酰化得到n-酰基苯丙氨酸,然后将其与基于聚乙二醇的胺 Jeffamine M1000 偶联,以产生最终的表面活性剂。使用该 FM1000 系列,观察到 14 碳长尾表面活性剂 (14FM1000) 与尾部更长或更短的表面活性剂相比,在防止 IgG 聚集方面是最佳的。使用动态表面张力测定法和石英晶体微天平与耗散的组合,假设 14FM1000 能够通过快速吸附到表面上来防止 IgG 吸附,从而防止聚集。与所研究的其他表面活性剂相比,14FM1000 的初始吸附速度最快。短尾表面活性剂缓慢且不会明显吸附到表面上,从而允许 IgG 吸附。虽然长尾表面活性剂也吸附缓慢,允许IgG吸附和聚集,它们的平衡吸附很强。此外,14FM1000 是最可逆吸附的表面活性剂,可能会提高其快速解吸和吸附到瞬态表面的能力,从而保护每个新疏水表面的 IgG 并防止聚集。通过了解表面活性剂与蛋白质稳定性之间的构效关系,我们朝着更有效的未来表面活性剂设计迈进,以提高重要蛋白质疗法的稳定性和实用性。
更新日期:2020-11-02
中文翻译:

疏水性尾长变化对表面活性剂介导的蛋白质稳定的影响
最近,蛋白质疗法由于与传统小分子药物相比具有更高的选择性和更少的副作用而备受关注。尽管具有优势,但蛋白质制剂通常会因生产和储存过程中的聚集和变性而受到稳定性问题的困扰,这通常会导致有害的免疫反应。表面活性剂可用于通过防止蛋白质吸附到界面上或通过在溶液中形成保护结构来稳定和保护溶液中的蛋白质。在此,描述了详细的结构-活性关系研究,证明了疏水尾长在表面活性剂介导的模型治疗蛋白 IgG 的稳定中所起的作用。FM1000 系列源自表面活性剂支架,可轻松进行结构调制,通过简单的两步程序合成。首先,苯丙氨酸用各种不同长度的酰氯酰化得到n-酰基苯丙氨酸,然后将其与基于聚乙二醇的胺 Jeffamine M1000 偶联,以产生最终的表面活性剂。使用该 FM1000 系列,观察到 14 碳长尾表面活性剂 (14FM1000) 与尾部更长或更短的表面活性剂相比,在防止 IgG 聚集方面是最佳的。使用动态表面张力测定法和石英晶体微天平与耗散的组合,假设 14FM1000 能够通过快速吸附到表面上来防止 IgG 吸附,从而防止聚集。与所研究的其他表面活性剂相比,14FM1000 的初始吸附速度最快。短尾表面活性剂缓慢且不会明显吸附到表面上,从而允许 IgG 吸附。虽然长尾表面活性剂也吸附缓慢,允许IgG吸附和聚集,它们的平衡吸附很强。此外,14FM1000 是最可逆吸附的表面活性剂,可能会提高其快速解吸和吸附到瞬态表面的能力,从而保护每个新疏水表面的 IgG 并防止聚集。通过了解表面活性剂与蛋白质稳定性之间的构效关系,我们朝着更有效的未来表面活性剂设计迈进,以提高重要蛋白质疗法的稳定性和实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号